Introduction
Behçet’s disease (BD) is a systemic vasculitis presenting with recurrent oral and genital aphthosis and some other organ involvement including eye, vascular, skin, joint, and neurological manifestations [1] with an annual incidence of 0.6% in Silk Road countries [2].
The exact aetiology of BD is not well understood yet; some studies showed that genetic and environmental factors play a role. Familial aggregation, higher prevalence in carriers of HLA-B51/5 and some other genes like tumour necrosis factor, heat shock proteins (HSP), and major histocompatibility complex class I show the role of genetic factors. In addition, exposure to some infectious agents, especially to members of the Streptococcus family, has been found to play a role [3, 4].
The HSP65 gene is linked with Streptococcus sanguinis (S. sanguinis), which is the most common isolated type of bacterial flora from the oral cavity of BD patients and may play a role in disease pathogenesis. It has a similar structure to human HSP60. It has shown that S. sanguinis and HSP 60/65 kDa can activate γδT cells in BD patients but not in controls. So, it seems that after the bacterial stimulus, mucous cells express HSPs which react with anti-mucous T cells in susceptible individuals (molecular mimicry) [5]. Recent studies also suggest that differences in the salivary or gut microbiome composition of these patients may also play a role [6].
Isolated recurrent aphthous stomatitis (RAS) is a benign and prevalent disease that can affect about 11–20% of the general population. It is mostly idiopathic and multifactorial. It can be the first presentation of patients with BD. A triad of RAS, relapsing uveitis, and genital ulceration is characteristic in BD [7].
The skin pathergy test (PT) is used as a criteria for the diagnosis of BD according to the International Study Group (ISG) criteria for Behçet’s Disease [8] and the Revised International Criteria for Behçet’s Disease (ICBD) [9]. A positive PT is defined as an erythematous induration at the site of the needle stick [10], appearing after 24–48 h as an erythematous papule or pustule [11]. The positivity rate of PT has been reported as 57.4% in Iranian, 44% in Japanese, 40% in Korean, 56% of Turkish, 68% in Moroccan, and 32% in British patients with BD [12].
It is believed that PT is a hypersensitivity reaction to skin streptococcal antigens penetrating into the skin during the skin prick test [13]. Given the oral cavity hygiene and mouth normal flora containing S. sanguinis as the most frequent organism, a relationship between this microorganism and positivity of skin PT is discussable; S. sanguinis is not only present in the mouth, but also it could be found as a prevalent normal flora of the skin [13].
The low sensitivity of PT, which is used in diagnosing BD patients, can probably be attributed to the development of hygiene facilities in society, leading to a decrease in S. sanguinis on the skin.
Davatchi et al. [14] revealed a significant decrease in test sensitivity from 64.2% to 35.8% over 35 years in the Iranian population. Multiple factors have been suggested that can increase test positivity, including using 2 skin prick tests [15], multiple puncturing [16], use of a blunt needle [17], resting the needle in the dermis for 90 seconds [18], the diameter of the needle [19], avoidance of surgical cleaning of the skin with antiseptic povidone-iodine (PVP-I) before insertion of the needle [20], and the use of neat self-saliva (as done recently by Mohamed et al. using a sample of fresh saliva diluted with water and then sterilized using a filter paper) [21, 22].
Due to the decrease in the positivity of the PT over recent years, we conducted this study to find a more sensitive and less invasive tool using a single dry 23G needle, a single self-saliva coated 23G needle, compared to the most routine and recommended 20G needle; also, we aimed to evaluate their positivity and diagnostic accuracy in differentiation of patients with active BD, RAS, and a control population.
Material and methods
We included 33 patients with BD, who fulfilled the ICBD criteria [9]; they consisted of 20 patients newly diagnosed with active disease and 13 inactive patients who referred to the rheumatology clinic of Motahari, as a tertiary health care centre affiliated with Shiraz University of Medical Sciences as a referral centre for patients with BD; also, we included 20 patients with RAS and 34 age- and sex-matched healthy controls from July 2019 to November 2020.
The patients with RAS should have a confirmed history of recurrent oral aphthosis with a history of at least one single oral aphthous in the last 2 weeks of referral, be in good condition with no serious systemic disease, and not fulfil the ICBD criteria for BD. They were referred to our BD clinic for complete evaluation of the presence of BD.
Disease activity was evaluated by the BDCAF index [23], so that one point was given to each new sign or symptom of different organ involvement (with total of 12 points) thought to be due to BD during the last 4 weeks prior to assessment, and the patients were considered active disease with BDCAF score ≥ 1. Inactive patients with BD had BDCAF = 0 (which means no new change over the last 4 weeks before the day of evaluation).
The exclusion criteria in patients with active BD, RAS, and the control group were:
patients who received antibiotics, glucocorticoids, or immunosuppressants and colchicine within 2 weeks prior to the study,
all patients with periodontal or dental treatments that interfered with the results of our study,
patients with proven inflammatory bowel disease, malignancy, or active infection and patients who were using anticoagulants at the time of evaluation.
Written consent forms were taken from each participant. This study was approved by the institutional review board of Shiraz University of Medical Sciences and received the approval of the Ethics Committee.
A hairless and avascular area on the right and left forearms of all groups was cleaned with ethyl alcohol. In the left forearm, the test was done with a 23G needle soaked with fresh self-saliva intra-dermally. We put the needle under the patient’s tongue and filled it with saliva, which took 30 seconds, keeping the tongue upward in the mouth. We used a vertical approach with a depth of 5 mm, lasting for 30 seconds, and then we did a 360-degree circular turn before taking the needle out of the skin. Then, at a distance of about 10 cm another PT was done using a 23G dry needle without saliva with the same depth and rotation as described before. In the right forearm, a single test was done as 20G PT using a 20G dry needle again with the same depth and rotation described before [24].
The sites of the tests were marked and checked after 48 hours by the rheumatologist, and an image was obtained for further evaluation. All tests were scored between 1+ and 4+ (0: only a needle mark, 1+ = 2 mm papule, 2+ = 2–4 mm papule, 3+ = ≥ 4 mm papule, and 4+ = pustule formation), and scores ≥ 2+ were labelled as positive for all tests [24].
Descriptive statistics including mean and standard deviation for quantitative variables as well as frequency and percentage for qualitative variables were used to describe the data. Data analysis was conducted using the Mann-Whitney U test for continuous, and the chi-square or Fisher’s exact test for categorical variables. Also, receiver-operating characteristic (ROC) analysis was used for diagnostic tests.
All tests were two-tailed, and p-values < 0.05 were considered statistically significant. Receiver-operating characteristic (ROC) analysis extends the assessment of test performance by providing information about all possible pairs of achievable sensitivity and specificity values. Statistical analyses were performed using SPSS version 18 (IBM Corp., Armonk, NY, USA).
Results
In this study, there were 20 patients with active BD, 20 with RAS, 13 with inactive BD, and 34 healthy population. The mean BDCAF during 4 weeks before evaluation was 1.80 among active BD with a frequency of oral aphthosis 14 (70%), ocular 15 (75%), genital ulcer 5 (25%), skin lesion (erythema nodosum) 1 (5%), and arthritis 1 (5%).
The demographic data and positivity of the characteristics of different PTs of all the groups are shown in Table I, and Figs. 1, 2.
Table I
Characteristics of patients with active Behçet’s disease, recurrent aphthous stomatitis, inactive Behçet’s disease, and the control group and the positivity of the standard 20G PT (test 1), PT using dry 23G needle (test 2), and PT with saliva using a 23G needle (test 3) in these groups
| Participants | Active BD | RAS | Inactive BD | Control | p-value |
|---|---|---|---|---|---|
| Total number | 20 | 20 | 13 | 34 | |
| Mean age | 37.40 ±10.87 | 39.25 ±7.94 | 47.53 ±11.19 | 40.11 ±10.30 | 0.045 |
| Gender | |||||
| Female | 13 (65%) | 10 (50%) | 12 (93%) | 19 (56%) | 0.012 |
| Male | 7 (35%) | 10 (50%) | 1 (7%) | 15 (44%) | |
| Standard PT positivity (test 1) | 4 (20%) | 2 (10%) | 0 (0%) | 0 (0%) | 0.028 |
| PT with dry 23G needle positivity (test 2) | 4 (20%) | 2 (10%) | 0 (0%) | 0 (0%) | 0.028 |
| PT with saliva positivity (test 3) | 14 (70%) | 8 (40%) | 4 (30%) | 0 (0%) | 0.0001 |
| p-value* | 0.004 | 0.068 | 0.111 | ||
Fig. 1
Pictures of the forearms of 2 patients with active Behçet’s disease (active oral aphthous and active uveitis) 48 hours after standard pathergy test (PT) using 20G dry needle on the right side and PT with a 23G dry needle on the left lower forearm and a PT with 23G needle using self-saliva on the left upper forearm showing positive test only by self-saliva PT.
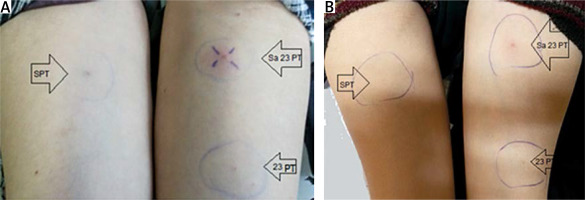
Fig. 2
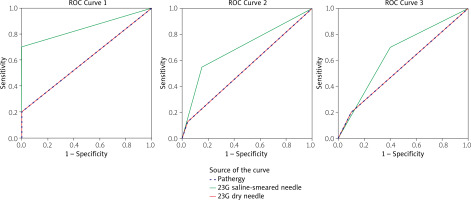
Receiver-operating characteristic curves for discrimination between: subjects with Behçet’s disease and recurrent aphthous stomatitis using pathergy test (PT), skin prick test using 23G needle with and without self-saliva (1), subjects with Behçet’s disease and controls using PT, skin prick test using 23G needle with and without self-saliva (2), subjects with Behçet’s disease (active + inactive) and non-Behçet’s disease patients (recurrent aphthous stomatitis + controls) using PT, and skin prick test using 23G needle with and without self-saliva (3).
In BD patients, the 20G PT and dry 23G needle PT were positive in 12.12% of all (active + inactive) the patients, and the self-saliva PT was positive in 54.55% of them. In the control group, results of all 3 PTs were negative. In addition, all 3 PTs were more positive among patients with active BD compared to RAS, inactive BD, and the control group (p-value = 0.028, 0.028, and 0.0001, respectively).
The same results were obtained for test 1 using a dry 20G needle and test 2 using a dry 23G needle in all groups, so for simplicity in interpreting the results, we performed these 2 tests as a single 20G test and compared them to test 3, which was PT using a 23G needle with self-saliva.
A comparison of the positivity of PT with saliva to 20G PT in each group showed that in active BD, PT with saliva was significantly more positive (p = 0.004). However, the positivity of this test in RAS (p-value = 0.068) and in inactive BD patients was not greater (p-value = 0.111).
A comparison of the active BD group to inactive BD patients revealed that 20G PT and PT with self-saliva were significantly higher in active BD patients (p-value 0.029 and 0.020, respectively).
Among the active BD group, patients with positive 20G PT had a mean BDCAF of 2.5, and those with positive PT with saliva had a mean BDCAF of 1.92, which means that PT with self-saliva is more sensitive than 20G PT in patients with lower disease activity (p-value = 0.046).
Then, we evaluated the diagnostic accuracy of 20G PT (Table II) and PT with self-saliva (Table III) in discriminating between BD patients and non-BD patients.
Table II
Diagnostic accuracy of standard pathergy test in discriminating between Behçet’s disease patients and non-Behçet’s disease participants
Table III
Diagnostic accuracy of pathergy test with self-saliva in discriminating between Behçet’s disease patients and non-Behçet’s disease participants
Figure 2 shows that the diagnostic accuracy of 20G PT in discriminating between active BD and the control group was significant (AUC: 0.600, p = 0.029) with a PPV of 100% and NPP of 80.7%, but it was not significant for discriminating the patients with BD (active + inactive) from non-BD (AUC: 0.542, p = 0.183); also, between active BD and the RAS group (AUC: 0.550, p = 0.383) it was not significant.
Then, we evaluated the diagnostic accuracy of PT self-saliva in discriminating between BD patients and non-BD patients, as shown in Table III. Diagnostic accuracy of PT with self-saliva was significantly high for discriminating patients with BD from non-BD and active BD from the controls (AUC: 0.699, p < 0.001 and PPV: 100%) and (AUC: 0.850, p < 0.001), respectively. Also, for discrimination between the patients with active BD and RAS, its diagnostic accuracy was close to significant (AUC: 0.65, p-value = 0.051).
The PPV of 20G PT and PT with self-saliva both were high (100%) in discrimination between active BD and the control group. In our studies, 8 (9%) participants experienced mild to moderate pain a few hours after PT with saliva (< 12 hours), which was spontaneously relieved, and only one patient developed hotness and swelling at the site of PT with saliva, which was relieved one day after treatment with cephalexin.
Discussion
The current work investigated PT using 3 methods: the most routine and recommended PT using a dry 20G needle, a 23G dry needle, and a wet 23G needle using self-saliva, to find the positivity, diagnostic accuracy, PPV, and NPP of these tests in patients with BD, with RAS, and a control group; we found similarity between dry 23G PT with 20G needle test as a smaller noninvasive tool and the higher sensitivity of self-saliva PT with a 23G needle for finding the patients with BD from non-BD groups.
In our study, all tests were negative in normal healthy controls, although in a previous study by Mohamed et al. [21], which compared PT and neat self-saliva PT in 30 patients with BD, 30 patients with RAS, and 30 healthy controls, the 20G PT was positive in 7% of the controls. In a study by Togashi et al. [22], none of the control group developed positive PT with neat self-saliva or with filtered self-saliva.
In our BD group (active and inactive), the 20G PT was positive in 12.12% and PT with self-saliva was positive in 54.5% of them. The positivity of this test in different countries in patients with BD ranged from 8.6% in India to 70.7% in China [25]. In Iran, the sensitivity of PT decreased from 64.2% to 35.8% during a 35-year period [14].
In our study, the positivity and diagnostic accuracy of 23G dry needle PT was the same as 20G PT, which was in contrast to previous studies that showed higher positivity of the test using thicker needles [19, 26].
After dividing the patients to active and inactive BD, we had no positive test using 20G PT and dry 23G PT in our inactive BD patients, although the PT with self-saliva was positive in 30% of the inactive BD group. Mohamed et al. [21] found 50% sensitivity of 20G PT among BD patients with inactive disease, who were not receiving steroids or immune suppressive drugs 2 week prior to the study, so probably the low number of our patients with inactive disease and also taking immunosuppressant or steroid medications was the reason for this negativity of the tests.
It should be considered that in previous studies it was shown that using the steroid treatment in patients with BD was not an interfering factor on PT positivity [27], although one study suggested a possible interfering effect of steroid usage on the PT of patients with BD [14].
In our study on active BD patients, the positivity of PT with self-saliva was significantly higher (70%) compared to 20G PT (20%), and it was also more positive among patients with lower disease activity than 20G PT, so it was more sensitive for active BD patients even when the activity was lower compared to 20G PT.
The study by Togashi et al. [22] showed a positivity of 90% using PT with neat self-saliva on 10 BD patients and the study of Mohammad et al. also showed positive PT with neat self-saliva in 83% (25/30) of patients with BD [21].
In our study, all tests showed higher positivity in patients with active disease compared to inactive ones. Some previous studies have shown that the results of the PT have no association with disease activity [28], although it was also shown that the positivity was lower in milder cases of BD [10].
In our study, the 20G PT and self-saliva PT were also positive (10% and 40%, respectively) in patients with RAS, with no significant difference (p-value = 0.068), while Mohammad et al. [21] showed positive PT with neat saliva in 37% (11/30) of the RAS group (although the description of a positive test was somewhat different). The Togashi et al. [22] study showed a weak reaction (mild erythema) with neat saliva PT in 60% (3/5) of the RAS group
We showed high diagnostic accuracy for 20G PT and PT with self-saliva in discriminating patients with active BD from the control group, and additional high diagnostic accuracy of PT with self-saliva for discriminating the patients with BD from non-BD, and nearly significant accuracy for discrimination between the patients with active BD and RAS.
The specificity of 20G PT and self-saliva PT were both 100% in comparison of active BD patients with the control groups. One study previously showed that the 20G PT had a specificity of 98.4% [14]. The specificity of the 20G PT test and self-saliva in our study in the discrimination of active BD patients from the RAS group was 90% and 60%, respectively, although none of the tests had significant diagnostic accuracy in determination of active BD patients and RAS participants.
We also did not follow up our patients with RAS to find the percentage that may evolve into BD over time, which may explain the positivity of self-saliva PT in 40% of our RAS patients.
The limitation of our study was the low number of patients and lack of follow-up study on patients with RAS. Therefore, further studies are recommended on a higher number of patients with BD using this more sensitive test and the relationship of its positivity to activity of the disease.
Conclusions
The pathergy test with self-saliva using a thin (23G) needle was more sensitive with equal specificity in detecting patients with BD from the control group, and it was more sensitive but less specific in detecting patients with BD compared to the RAS group than 20G PT.
The pathergy test is also more correlated to the disease activity compared to 20G PT. In addition, the accuracy of 20G PT is the same as dry PT with a 23G needle, so we can replace the 20G PT with a thinner 23G needle.



