Introduction
Behçet’s disease (BD) presents as a systemic vasculitis, characterized by a diverse array of symptoms impacting multiple bodily systems including the skin, mucous membranes, joints, eyes, blood vessels, nervous system, and gastrointestinal tract [1]. Unlike a persisting disease trajectory, BD typically manifests as an unpredictable pattern of relapses and remissions [2]. This erratic course can make managing the condition challenging for both patients and healthcare providers, as symptoms may vary in severity and frequency over time. The multisystem nature of BD underscores the complexity of its pathophysiology and the importance of comprehensive management strategies tailored to individual patient needs.
Assessing disease activity is crucial in the management of BD. The primary tools for evaluating BD disease activity are validated clinical instruments that focus on examining organ involvement and, to a limited extent, certain laboratory data with low specificity including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) [3]. Clinical assessment continues to play a vital role in evaluating BD activity, given that a definitive instrument has not been established despite extensive research into potential biomarkers. Biomarkers derived from routine laboratory tests that accurately reflect BD disease activity could hold significant practical value within clinical settings.
Lately, there has been a growing trend to utilize ratios of complete blood count (CBC) subparameters to albumin for assessing inflammation; it seems to be a practical, cost-effective, and routine testing method. Previous investigations have demonstrated that some of these ratios were related to the prognosis of many types of malignancies [4–6]. These ratios have also been studied as potential markers for disease activity in various autoimmune and rheumatic diseases [7–9]. Furthermore, CRP to albumin ratio (CAR) has been demonstrated to correlate with disease activity in certain rheumatological conditions [10–13]. However, to the best of our knowledge, no previous study has assessed the potential of these biomarkers in BD.
The primary aims of our investigation were to assess the potential of monocyte to albumin ratio (MAR), neutrophil to albumin ratio (NAR), platelet to albumin ratio (PAR), and C-reactive protein to albumin ratio (CAR) as disease activity biomarkers in patients diagnosed with BD and to evaluate their discriminative ability between cases and healthy controls.
Material and methods
Patients and study design
The inclusion criteria for patients were age ≥ 18 years and meeting the BD classification criteria [13]. The control group consisted of demographically similar healthy participants without known diseases. Exclusion criteria included individuals who were pregnant or lactating, those with concurrent inflammatory or autoimmune disorders, and individuals with infectious diseases, hematological disorders, or malignancies.
Based on CAR, a sample size calculation resulting in 90 participants with 90% statistical power and 95% reliability, the study included 45 healthy controls and 45 consecutive patients diagnosed with BD [3, 14].
Evaluations
Demographic characteristics (age, sex), disease duration (in months), and current medications for BD were documented.
To gauge the clinical disease activity in BD, we utilized the Behçet’s Disease Current Activity Form (BDCAF) [15, 16], which examines the clinical symptoms experienced by patients in the four weeks leading up to the assessment. The form has been appropriately validated in our language [17]. Each component in this evaluation is scored as either absent (0) or present (1). The final BDCAF score is calculated by adding up the positive components, with a maximum achievable score of 12. Active BD is defined by a BDCAF score ≥ 2, while a score < 2 indicates inactive BD, based on the outlined criteria [18].
In terms of laboratory evaluations, CBC, ESR (mm/h), CRP (mg/l), and serum albumin levels were assessed by standard laboratory procedures. Based on the concurrent CBC results of the participants, MAR, NAR, PAR, and CAR were calculated.
Statistical analysis
The data were subjected to statistical analysis using IBM SPSS Statistics for Windows 22.0 software. To examine data normality, the Shapiro-Wilk test was utilized. Descriptive frequencies were computed for both demographic and clinical variables. Categorical variables underwent analysis using the χ2 test, while continuous variables were assessed using either the Mann-Whitney U-test or Student’s t-test. Correlation analysis employed the Spearman test, with weak correlation denoted by r = 0.1–0.3, moderate correlation by r = 0.3–0.5, and strong correlation by r = 0.5–1.0. Receiver operating characteristic (ROC) curves were generated to determine cut-off points for laboratory parameters. A significance level of p < 0.05 was considered for statistical significance.
Bioethical standards
This cross-sectional study was conducted at Atatürk University School of Medicine Rheumatology Clinic from April 2023 to September 2023, adhering to the principles outlined in the Declaration of Helsinki. The study protocol obtained approval from the local Ethics Committee (approval number: B.30.2.ATA.0.01.00/185), and informed consent was obtained from all participants involved in the study.
Results
Our study comprised 45 individuals with BD (22 females, 23 males) and 45 healthy controls were included (22 females, 23 males). The demographic features were similar between groups. Thirty patients had active (66.7%), and 15 patients had inactive BD (33.3%). The mean ±SD of ESR, MAR, PAR, CAR, and albumin were significantly different between patients with BD and controls (p = 0.008, p = 0.009, p = 0.029, p = 0.034, p = 0.006, respectively). However, we did not observe a significant difference in CRP and NAR levels between groups. The demographic, clinical, and laboratory characteristics of the participants are outlined in Table I. No significant difference was observed in parameters such as CRP, MAR, NAR, PAR, and CAR between patients with active BD and those with inactive BD, except for ESR (13.46 ±9.97 and 6.86 ±5.22, respectively for active and inactive BD, p = 0.03).
Table I
Demographic, clinical and laboratory characteristics of participants
[i] BD – Behçet’s disease, BDCAF – Behçet’s Disease Current Activity Form, CAR – C-reactive protein to albumin ratio, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, MAR – monocyte to albumin ratio, NAR – neutrophil to albumin ratio, PAR – platelet to albumin ratio, TNFi – tumor necrosis factor inhibitor.
In correlation analysis, no significant correlation was found between BDCAF and ESR, CRP, MAR, NAR, PAR, or CAR (for ESR; p = 0.09, r = 0.25, for CRP; p = 0.069, r = 0.06, for MAR; p = 0.06, r = –0.282, for NAR; p = 0.62, r = –0.07, for PAR; p = 0.13, r = 0.22, and for CAR; p = 0.69, r = –0.05).
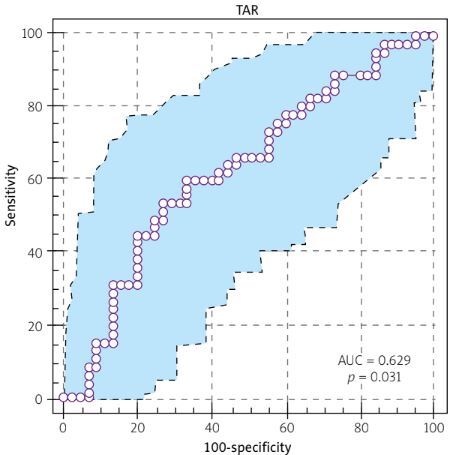
We further assessed the discriminatory capacity of MAR, NAR, PAR, and CAR in distinguishing BD cases from the controls. Cut-off values were determined using the Youden index, which maximizes the sum of sensitivity and specificity. The cut-off point for MAR was established as 150.59 (sensitivity 46.67% and specificity 82.22%, p = 0.008, AUC = 0.655), for PAR as 62,013.73 (sensitivity 60.00% and specificity 66.67%, p = 0.03, AUC = 0.629), and for CAR as 1.16 (sensitivity 35.56% and specificity 95.567%, p = 0.03, AUC = 0.629). However, no statistically significant cut-off value was determined for NAR (Fig. 1). Also, the results were not able to define any cut-off value to distinguish patients with active and inactive BD in MAR, NAR, PAR, or CAR.
Discussion
The objective of the current study was to assess MAR, NAR, PAR, and CAR as potential biomarkers of disease activity in individuals diagnosed with BD. Our results demonstrated significantly higher levels of these parameters in patients with BD than controls except NAR. Furthermore, our study was able to define cut-off points for MAR, PAR, and CAR for their discriminatory ability to distinguish patients with BD and controls. However, no significant difference was found in MAR, NAR, PAR, and CAR between cases of active BD and cases of inactive BD, and no significant correlation was found between disease activity score and these parameters.
Assessing disease activity in BD is a crucial responsibility for clinicians in their daily practice, as well as a necessity for conducting clinical research. To date, there is no dedicated laboratory test for diagnosing BD, and no single biomarker has been universally acknowledged as a specific method to identify and accurately estimate BD activity. Presently, assessing BD activity primarily relies on clinically evaluating the involvement of various organs and systems. Integrating potential biomarkers alongside clinical indices will aid physicians in evaluating BD activity more effectively.
In daily practice, the most commonly utilized tests are ESR and CRP [19]. These tests have been extensively investigated in clinical practice through various studies, yielding results that are sometimes inconsistent with each other [20]. A previous study on BD indicated a connection between higher levels of ESR and CRP and the presence of articular involvement, superficial thrombophlebitis and erythema nodosum [21]. The same study also identified a correlation between BDCAF score and ESR and CRP values. In our present study, notably elevated levels of ESR and CRP were determined in patients with BD compared to the control subjects. Furthermore, ESR levels were notably higher in BD cases with active disease compared to those with inactive disease. However, our investigation did not reveal a significant correlation between BDCAF score and these parameters.
The immune cells, such as neutrophils, monocytes, and platelets, may serve as predictors of the progression of systemic inflammation. Also, high CRP and low albumin are well known to be associated with inflammation. From this point of view, in recent years, as practical and cost-effective methods, the ratios of CBC sub-parameters and CRP to albumin have been shown to have predictive value in prognosis and disease activity in several malignant, autoimmune and rheumatological diseases [4, 5, 8, 10–12]. In previous studies, PAR was found to be elevated in cases of rheumatoid arthritis (RA) and increased with the disease activity [22]. Furthermore, PAR, MAR, and NAR were investigated in axial spondylarthritis and PAR was found to be a reliable indicator of disease activity in these patients [23]. In ANCA-associated vasculitis (AAV), CAR has been identified as an independent predictor for all-cause mortality among patients [11]. Additionally, a separate study noted a significant association between CAR and disease activity in AAV, suggesting its potential utility as a tool for monitoring disease activity [24]. In terms of BD, CAR and its association with BDCAF scores and uveitis in these cases have been studied very sparsely [3, 13]. In the current investigation, significantly higher levels of MAR, PAR, and CAR were observed in patients with BD than controls, with their discriminatory ability. However, we were unable to establish a significant correlation between these parameters and disease activity in BD, and no significant difference in these parameters was found between the active and inactive patient groups. Our data do not support the potential of these parameters as biomarkers of disease activity in BD. This also may be due to the heterogenous nature of BD, which makes it difficult to generate a certain single score for disease activity.
Considering our results together with previous data, the ratios of CBC sub-parameters and CRP to albumin appear to be informative, simple, practical and cost-effective tools which may be supporting biomarkers of the disease activity in BD. Despite acknowledging limitations such as infections and thrombocytopenia [25], these parameters may serve as practical and informative indicators of disease activity in BD.
Study limitations
The limited number of cases of inactive BD was the main limitation of the current study. This may be due to the fact that most patients were under immunosuppressive treatment in our study. Also, since this may be due to the study center’s tertiary nature, multicenter study designs including more cases may be needed.
The primary strength of this study lies in its pioneering investigation of MAR, NAR, PAR, and CAR as potential biomarkers of disease activity in patients with BD.
Conclusions
Our study revealed significantly higher levels of MAR, PAR, and CAR in BD patients than controls. No significant difference was found in MAR, NAR, PAR, and CAR between cases of active BD and cases of inactive BD. These parameters, by demonstrating their discriminative capacity, could potentially function as biomarkers to aid in clinical assessments of BD.