Introduction
Clinical manifestations of systemic vasculitides share many common features with other conditions, this being the cause of diagnostic errors, delayed diagnosis and treatment, and even increased mortality [1]. In seventy-three percent of cases the initial diagnosis appears to be wrong; in particular, in 33% of patients systemic vasculitis is misdiagnosed as infection, in 5% as cancer, and in 19% as another autoimmune disease [1].
Therefore, many practicing medical researchers recommend considering anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) as a differential diagnosis for patients with atypical manifestations of malignancies (including suspected solid tumors and hematologic malignancies). Data regarding lung cancers with coexisting systemic vasculitis with pulmonary manifestations are quite limited [2].
Material and methods
The relevant literature describing differential diagnosis of systemic vasculitis and lung cancer has been reviewed. We searched PubMed, Medline, Scopus and Web of Science databases using the following combinations of words: systemic vasculitis, lung cancer mimics, granulomatosis with polyangiitis (GPA), delayed diagnosis.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for Individual Patient Data systematic reviews (PRISMA-IPD) was used [3].
The search retrieved 14 results (Fig. 1). Clinical case reports demonstrating various patterns of pulmonary involvement were analyzed: cases of granulomatosis mimicking lung cancer, lung cancer masquerading as granulomatosis, and different variants of coexistence of systemic vasculitis and lung cancer (Table I).
Table I
Overview of selected studies with summary of key findings
| Study (author and reference) | Study design | Study population/material | Key findings |
|---|---|---|---|
| Sreih et al. [1] | A survey of open-ended questions about patients’ diagnostic journeys and perceived factors associated with rapid or delayed diagnosis | 456 patients with systemic vasculitides | 73% of patients with vasculitis were initially misdiagnosed and had negative consequences for their health |
| Urbanska et al. [2] | A single-centre case report | 1 patient | A report of an uncommon presentation of GPA mimicking metastatic lung cancer |
| Li et al. [6] | A retrospective analysis of computerized records | 45 patients with GPA | CT manifestations of GPA were initially misdiagnosed as pneumonia, tumours and other diseases in 53% of patients; CT scans combined with clinical and laboratory data and biopsy may be required for a definite diagnosis |
| Ananthakrishnan et al. [7] | Literature review | 15 references | Active GPA can mimic pneumonia, septic emboli and metastases |
| Hesford et al. [8] | A case report and literature review | 1 patient and 10 references | A report of GPA with unusual dural and aortic and pulmonary trunk involvement mimicking multifocal adenocarcinoma |
| Feragalli et al.. [9] | Literature review | 25 references | Since the diagnosis of vasculitis is often delayed because several other diseases have similar clinical manifestations, this review aims to correlate radiological findings with pathology results |
| Taimen et al. [15] | A single-center study | 317 patients with systemic vasculitis | The authors detected a substantial diagnostic delay in tertiary-level health care when diagnosing systemic vasculitis. This delay is positively correlated with costs during the first year of care and costs in a tertiary health care facility |
| Uppal et al. [16] | A single-center case report | 1 patient | The authors describe a patient with a probable GPA which was originally diagnosed as malignancy, but who responded to lung cancer chemotherapy |
| Xie et al. [19] | A single-center case report | 1 patient | A case reporting the coexistence of vasculitis and squamous cell carcinoma in the same cavitating lung mass and highlighting the importance of early recognition of changes in the disease course in order to provide timely management |
| Masiak et al. [20] | A case report and literature review | 1 patient and 58 references | The authors discuss the risk of the development of lung cancer in GPA. Despite symptoms suggestive of exacerbation of vasculitis, such as a new mass and a high level of ANCA, it is still necessary to consider other causes of pulmonary masses as well as to consider the carcinogenic potential of drugs used to treat GPA |
| Chemouny et al. [21] | A case report series | 5 patients | Report of 5 patients with ANCA-associated diseases who had associated lung cancer or were diagnosed with lung cancer within 2 years after vasculitis onset |
| Hutson et al. [22] | Retrospective review of patients’ records | 2,800 patients | The observed close temporal relationship of cancer and vasculitis, in authors’ opinion, adds to evidence of vasculitis at times being a paraneoplastic condition. Failure of a vasculitis to respond to conventional therapy should raise questions about underlying malignancy |
| Zhang et al. [23] | A single-centre case report | 1 patient | This is the first published report of lung adenocarcinoma and GPA |
| Do et al. [24] | A single-centre case series | 73 patients | The frequency of cancer-associated vasculitis was 0.034% in Korean patients. The types of cancers and vasculitides in cancer-associated vasculitis and the distributions of gender and age may be dependent on ethnic and geographic differences |
Fig. 1
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of searching methods.
Case description
A 61-year-old female patient with a 10-year history of hypertension presented to patient’s family physician as hypertension became uncontrolled despite the medications used. The patient also reported frequent hypertensive crises and nosebleeds. During examinations a high creatinine level (232 mmol/l) was revealed and the patient was consulted by a nephrologist who diagnosed stage III chronic kidney disease.
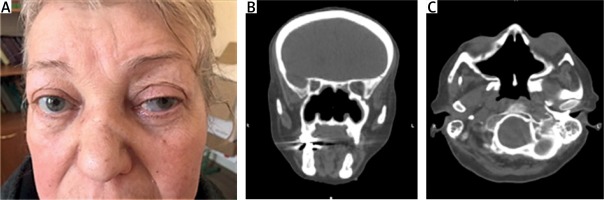
A year later, the kidney function deteriorated and nose deformity was also revealed, as well as difficult nose breathing, and frequent nosebleeds continued (Fig. 2A).
Fig. 2
Appearance of the patient at presentation: a photo demonstrating episcleritis, proptosis, nose deformity (A). The photo is used with the patient’s consent. Computed tomography images demonstrating osteolytic changes of nasal bones and of the walls of paranasal sinuses (B, C).
In the described patient an otorhinolaryngologist diagnosed a chronic bilateral maxillary sinusitis. Meanwhile the patient’s condition was worsening: patient developed progressive dyspnea, dysphonia, redness and loss of vision in the left eye, neurological symptoms (headaches, dizziness, ataxia, sensorineural hearing loss).
Head computed tomography (CT) and magnetic resonance imaging (MRI) were consistent with bilateral frontal sinusitis, ethmoiditis, sphenoiditis and mastoiditis. The two upper thirds of the nasal septum and the medial walls of the maxillary sinuses were absent (Figs. 2B and 2C).
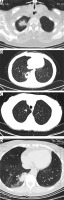
High-resolution computed tomography (HRCT) of the chest revealed peripheral (subpleural) masses in segments 1, 3 and 10 in the right lung, a cavitation, and small pulmonary polygonal nodules (Fig. 3).
Fig. 3
Computed tomography scans demonstrating a mass in segment 3 in the right lung (A), a cavitation in segment 10 (B), pulmonary nodules of polygonal shape with peribronchovascular and peripheral localization (C), and a mass in segment 10 of the right lung (D).
Also, CT and positron-emission tomography (PET)-CT detected infiltration of the upper third of the tracheal wall with tracheostenosis (Fig. 4).
Fig. 4
Computed tomography scans (A–C) and positron-emission tomography images (D–F) demonstrating narrowing of the trachea and subglottic stenosis (arrows).
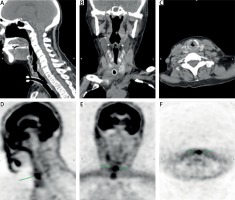
Indirect laryngoscopy confirmed the presence of a mass at the level of 1–2 tracheal rings obstructing the tracheal lumen. Transthoracic right lung biopsy was consistent with an infiltrative growth of a nonkeratinizing squamous cell carcinoma (G2). Computed tomography-guided Tru-cut biopsy of the right lung apical mass revealed large areas of connective tissue overgrowth with signs of focal fibrinoid necrosis.
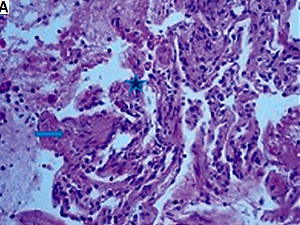
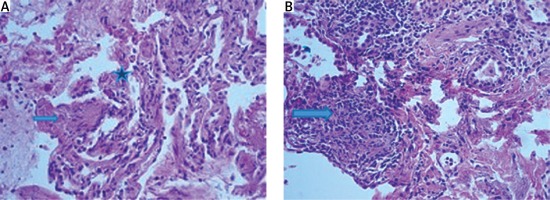
Small to medium-sized vessels appeared spastic with thickened walls due to fibrinoid, sclerotic changes and endothelial proliferation; vascular wall cellular infiltrates with karyorrhexis and perivascular fibrosis were noted. In one of the areas a multinucleated giant cell was found (Fig. 5A). The pattern of cellular infiltrates was suggestive of granulomatous inflammation (Fig. 5B).
Fig. 5
Lung tissue with accumulation of fluid, fibrin, and squamous cells in some of the alveolar spaces and focal carnification (asterisk). A multinucleated giant cell is seen (arrow). Hematoxylin and eosin staining, × 200 (A). Fibrosis of the interalveolar septa, infiltrated by lymphocytes, histiocytes/macrophages, plasma cells, eosinophilic leukocytes and monocytes. Hematoxylin and eosin staining, × 200 (B).
Subsequently, the patient underwent serologic screening for systemic vasculitides and was found to be strongly positive for ANCA in the IgG class of immunoglobulins directed to proteinase-3 (anti-PR3-ANCA).
Therefore, the patient was deemed as having a diagnosis of GPA according to the 2022 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) Classification Criteria for GPA [4] with a score of 16 points as well as 16 points according to the previous ACR/EULAR 2017 Provisional Classification Criteria for GPA [5].
In particular, according to the 2022 ACR/EULAR Classification Criteria for GPA the patient fulfilled the following criteria:
nasal involvement with bloody discharge, septal defect/perforation (3 points),
cartilaginous involvement with nose saddle deformity (2 points),
sensorineural hearing loss (1 point),
positive PR3-ANCA antibodies (5 points),
pulmonary nodules, mass, cavitation on chest imaging (2 points),
extravascular granulomatous inflammation and giant cells on biopsy (2 points),
inflammation of paranasal sinuses and mastoiditis on imaging (1 point).
The patient also fulfilled the following ACR/EULAR 2017 Provisional Classification Criteria for GPA:
bloody nasal discharge, sinonasal congestion (3 points),
cartilaginous involvement (2 points),
red eyes (1 point),
nodules, masses and cavitation on chest imaging (2 points),
granuloma on biopsy (3 points).
The patient was administered remission induction treatment with cyclophosphamide 200 mg i.v. every week and methylprednisolone 500 mg pulse therapy. Mycophenolate mofetil and methylprednisolone 1 mg/kg were used for maintenance of remission.
Discussion
Granulomatosis with polyangiitis is a rare multisystem disease with an incidence of 20 per million population per year [2]. It is characterized by small vessel vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tract and kidneys, and the presence of ANCA antibodies [6, 7]. We performed analysis of the available data regarding misdiagnosis of vasculitis as lung cancer and vice versa, as well as coexistence of vasculitis and lung cancer.
Initial manifestations of GPA are non-specific, and because of its rarity the correct diagnosis may be deferred for months or years in many cases [8–10]. Most often, otorhinolaryngologists and ophthalmologists are the first contact physicians of patients with GPA capable of recognizing pansinusitis and the systemic character of the disease.
However, the majority of current otorhinolaryngology protocols do not recommend screening for systemic vasculitides [11, 12], in contrast to some ophthalmological recommendations advising ANCA testing in selected cases [13, 14].
The average time span from the first referral to hospital to referral to the tertiary care center was found to be the shortest in patients with large vessel vasculitides and the longest among patients with AAV [15].
In the above-described case pulmonary nodules, cavitations and parenchymal densities were first misdiagnosed as lower lobe lung cancer with secondary dissemination. Transthoracic biopsy was erroneously interpreted as nonkeratinizing squamous cell carcinoma, while tracheal stenosis was also considered to be a paraneoplastic process.
This reemphasizes that the diagnosis of systemic vasculitis is indeed challenging due to the heterogeneity of clinical manifestations. Due to a thorough analysis of the patient’s history and of clinical multisystem manifestations, the suspicion of systemic necrotizing vasculitis was raised.
In fact, most of the publications discuss misdiagnosis of vasculitides as cancer [2, 16, 17]. Reports of coexistence of systemic vasculitis and lung cancer are scarce and limited to case reports [18–20] and small case report series [21, 22]: exposure to cyclophosphamide seems to be one of the main causes of development of lung cancer in patients with GPA, while a case report of a new onset GPA in a patient with known lung adenocarcinoma is unique [23].
On the other hand, in a recent single-center study, coexistence of cancer and vasculitis was found in 0.034% of patients with cancers: GPA was the second most frequent type of vasculitis associated with cancers after Behçet’s disease [24].
Of note, lung cancer ranked ninth among cancers associated with vasculitides, preceded by thyroid cancer (the most common type of malignancy), lymphoma, stomach cancer, breast cancer, brain tumors, leukemia, hepatocellular carcinoma and prostate cancer [24].
Overall, in patients with systemic disease a multidisciplinary team approach remains a cornerstone for interpretation of pulmonary involvement, planning further diagnostic steps in order to minimize the delay in diagnosis and treatment [25].
In the present case, CT imaging raised the suspicion of lung cancer, while further biopsies and laboratory testing rejected malignancy and confirmed the diagnosis of GPA.
Conclusions
Computed tomography findings in GPA may suggest lung cancer, sarcoidosis, tuberculosis, or lymphoproliferative disorders and require a thorough differential diagnosis.
Screening for ANCA and PR3-antibodies is important for differential diagnosis in patients with multisystem involvement. Therefore, we suggest ANCA and anti-PR3-ANCA antibody testing to be considered by otorhinolaryngologists, especially in young patients presenting with pansinusitis and hearing loss.