Introduction
Rheumatoid arthritis (RA) is known to be a chronic autoimmune disease characterized by progressive erosive destruction of peripheral joints [1].
An important component of the RA pathogenesis is dysregulation of the immune system, which induces impaired tolerance of autoantigens and the development of autoimmune reactions. Predisposition to RA development is determined by genetic, age and microenvironment factors. Establishing the functionally different T cellular subpopulations (Th1 and Th2) made it possible to elucidate the different roles of pro- and anti-inflammatory cytokines produced by them in the initiation and maintenance of long-term autoimmune diseases including RA [2].
Activation of specialized synoviocyte-like fibroblasts that produce the inflammatory mediators may be crucial for a cytokine profile alteration in RA resulting in disruption of the coordinated link between definite immune competent cells and developing immune inflammatory reactions in general [3].
The basic principle to treat this pathology should be the correction of cytokine profile and the interactions of immune competent cells.
According to the modern concepts, RA development is associated not only with differentiation, proliferation and activation of effector T cells, but also with impaired suppressor mechanisms that control tolerance susceptibility of T and B lymphocytes to autoantigens [4].
A key role in the mechanisms of immune suppression is played by regulatory T cells with CD4+CD25+ phenotype [5]. Patients with RA have a strongly reduced number and disturbed function of these cells. CD4+CD25+ T cells have been found to play a role in the understanding of regulatory mechanisms of autoimmunity, leading to RA development and progression [6].
Today, a wide range of immune modulators is used in the therapy of RA [7, 8]. These preparations are not always highly effective, and the results are sometimes contradictory and ambiguous. Important factors limiting the immunosuppressive therapy of RA include the resistance to previously effective drugs, which often occurs with their long-term use.
This emphasizes the need for further development of new effective and safe immunotropic agents for correction of the immune competent sphere of the patients with arthritis. In this regard, one cannot fail to note the many profiles of immune corrective activity of cell therapy.
One of the promising therapeutic approaches is the application of multipotent mesenchymal stromal cells (MMSCs) [9]. Having a high regenerative and reparative potential, these cells are able to realize the immune modulatory potential, including the immune suppressive one [10].
Bone marrow, adipose tissue, umbilical cord blood, and endothelial progenitor cells are the most popular types of MMSCs used for autoimmune disease treatment. Multipotent mesenchymal stromal cells have received a lot of interest because of their source potential, high proliferation rate, less invasive technique, and lack of ethical concerns compared to other therapies, and therefore they have a significant advantage. The immune suppressive and anti-inflammatory properties of MMSCs have led to these cells being tested for their therapeutic potential in preclinical models of RA such as inflammatory arthritis [11].
Several studies have suggested that bone marrow- or adipose-derived MMSCs have the ability to “reset” the immune system, reducing the deleterious Th1/Th17 response and enhancing the protective regulatory T cell response, although other studies have failed to demonstrate an improvement with MMSC treatment.
The inconsistent results in preclinical models may be due to several variables such as source of MMSCs (syngeneic, autogenic or allogenic), tissue of MMSC origin, timing of treatment, number of injected cells, route of injection and treatment regimes, different culture conditions, as well as differences in animal strains and their housing conditions.
Although the results of experimental and clinical studies in recent years have indicated the immune modulatory, healing, anti-inflammatory effects of MMSCs, the strength and duration of the effect are very different, which indicates an incomplete correction of the tested parameters, thereby opening up the prospect of improving this method of treatment by choosing dose-time parameters and methods of their administration.
The aim of the study was to determine the indices of cellular immunity in animals with adjuvant arthritis treated with cryopreserved MMSCs (CrMMSCs) derived from adipose and cartilage tissues.
Material and methods
Outbred white male rats (aged 12–14 weeks, n = 35) were used in the study. The rats were kept in plastic cages (five animals per cage) at a controlled temperature (18–22°C), humidity (30–70%) and lighting (light interval from 8.00 to 20.00) with free access to water and food according to the standard diet. The acclimation period was 7 days.
The suspension of cells from the rat’s adipose (AT) and cartilage (CT) tissues was obtained from biopsies by enzymatic treatment [12]. Subculturing was performed until AT and CT MMSCs cultures reached the monolayer, followed by their cryopreservation under 10% DMSO protection (Sigma-Aldrich, USA) with the addition of 20% fetal serum (HyClone, USA). The cooling rate was 1°C/min to –80ºC with subsequent immersion into liquid nitrogen [13].
Cryopreserved MMSCs were warmed in a water bath at 40ºC until the appearance of the liquid phase. The cryoprotectant was removed by adding Hanks’ solution (PAA, Austria) in a ratio of 1 : 9, followed by centrifugation at 250 g for 5 minutes.
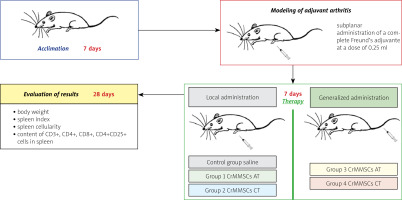
The adjuvant arthritis (AA) was modeled in male rats by subplantar administration of complete Freund’s adjuvant at a dose of 0.25 ml (Santa Cruz, USA). The chosen experimental model is similar to RA changes in humans: adjuvant-induced arthritis shares common symptoms such as joint swelling, lymphocyte infiltration and cartilage degradation [14].
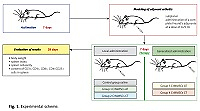
Cryopreserved MMSCs from adipose and cartilage tissues of rats were used in the research. The scheme of the experiment is shown in Figure 1.
On day 7 of adjuvant arthritis modeling, experimental animals were administered with:
control group (n = 5) – saline,
experimental group 1 (n = 5) – CrMMSCs from adipose tissue at a dose of 0.25 × 106 cells locally,
experimental group 2 (n = 5) – CrMMSCs from cartilage tissue at a dose of 0.25 × 106 cells locally,
experimental group 3 (n = 5) – CrMMSCs from adipose tissue at a dose of 0.5 × 106 cells generalized,
experimental group 4 (n = 5) – CrMMSCs from cartilage tissue at a dose of 0.5 × 106 cells generalized.
A group of intact animals (n = 5) was also formed.
On day 28 after therapy the body weight, spleen index, spleen cellularity, and content of CD3+, CD4+, CD8+, CD4+CD25+ cells in spleen were determined. The spleen index was evaluated according to the formula: organ mass/animal mass × 100. The index of spleen cellularity was calculated as the ratio of the number of cells in experimental animals’ spleen to the average number of cells in the organ of intact animals.
A cell suspension was obtained from the spleen of the animals of the study groups by homogenizing the organ in 5 ml of Hanks’ solution in a Potter homogenizer. The cell suspension was centrifuged (1,000 rpm, 10 min), and the supernatant was removed. Cell sediment was diluted with 2 ml of medium, pipetted and the number of nucleated cells was counted in a Goryaev chamber.
The number of individual subpopulations of immune cells in the spleen was determined by flow cytometry using monoclonal antibodies labeled with FITC (BD, USA) to CD3 (total T lymphocytes), CD4 (T helpers), and CD8 (T suppressors).
In addition, the immune regulatory index (IRI) was calculated from the ratio of T helpers and T suppressors (CD4+/CD8+). Monoclonal antibody to CD4 and CD25 membrane structures (BD Pharmingen, USA) was used to quantify Treg cells. Immunoglobulins of the same isotypes were used as an isotype control.
Cell fluorescence was assessed with a FACSCalibur flow cytometer (BD, USA). All procedures and manipulations with the cells were performed in the standard way according to the manufacturer’s instructions.
Statistical analysis
Data analysis was carried out using WinMDI 2.8 software (USA). At least 10,000 cells were analyzed in each sample. Significance of differences between the groups was assessed by the Mann-Whitney U test using Statistica 8 software (StatSoft Inc., USA). The critical value of the significance level was assumed to be 0.05.
Bioethical standards
Experimental studies were performed in compliance with the requirements of humane treatment of experimental animals, regulated by the Law of Ukraine “On the Protection of Animals Against Cruelty” (Approval date/no: 21.02.2006/3447-IV), the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986) and the Protocol of the Bioethics Committee of the Institute for Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine (no. 2018-01).
Results
The most obvious manifestations of AA in mice and rats are edema, deformity and impaired joint mobility, splenomegaly, decreased T suppressor immunity, and changes in the cytokine profile [15].
At the site of injection of complete Freund’s adjuvant, there were signs of joint inflammation (redness, swelling), which were clearly expressed in the control group throughout the experiment, and in the animals with cell therapy, these signs were fading.
The animals were monitored daily for health status during the whole investigation period, and adverse events were not observed. In intact animals, a gradual increase in body weight could be noted during the experiment, while in animals with AA these dynamics were smoothed out (Fig. 2).
Fig. 2
Effect of cryopreserved multipotent mesenchymal stromal cells from adipose and cartilage tissues on body weight of rats with adjuvant arthritis (x ±SD, n = 5).
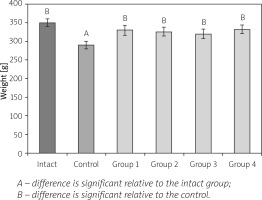
The control animals lost some weight after AA induction and as a result they lagged behind intact rats in weight (1.2-fold) at the end of the experiment. At the 28th day of observation the body weight of animals from experimental groups reached the level of intact rats.
Splenomegaly is another characteristic symptom of AA [16]. The effect of CrMMSCs from adipose and cartilage tissues on the spleen index and cellularity of rats with AA is presented in Figure 3 A, B.
Fig. 3
Effect of cryopreserved multipotent mesenchymal stromal cells from adipose and cartilage tissues on spleen index (A) and index of spleen cellularity (B) of rats with adjuvant arthritis (x ±SD, n = 5).
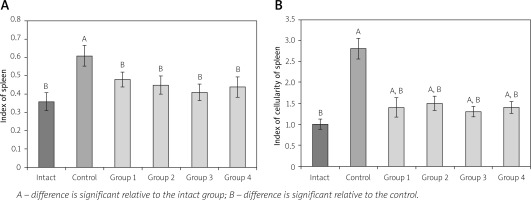
In animals of the control group an increase of the spleen index (1.7-fold) and cellularity (2.8-fold) of the spleen relative to the corresponding parameter value in the intact group was determined. This phenomenon is probably due to the prevalence of stromal element growth in the spleen with AA.
In animals with local administration of CrMMSCs from adipose and cartilage tissues (groups 1 and 2) at day 28, the spleen index was 1.27- and 1.36- fold lower than the corresponding value in the control group of animals (untreated AA). The studied indicator in groups 3 and 4 decreased respectively 1.49- and 1.38-fold compare to the control.
Spleen cellularity index values in animals from all experimental groups were lower than the corresponding values in the control group (on average 2 times). However, it should be noted that in all studied groups, the index of spleen cellularity did not reach the intact group value.
T cells and macrophages play a central role in the RA pathogenesis and progression [17, 18]. In the study, it was found that rats had changes in the quantitative parameters of immune competent cells in the spleen (Table I).
Table I
Influence of cryopreserved multipotent mesenchymal stromal cells from adipose and cartilage tissue on indices of cellular immunity of rats with AA (x ±SD, n = 5)
| Groups | CD3+ [n (%)] | CD4+ [n (%)] | CD8+ [n (%)] | CD4+/CD8+ |
|---|---|---|---|---|
| Intact | 42.9 ±0.4b | 36.5 ±0.5b | 23.7 ±0.3b | 1.54 ±0.13b |
| Control | 20.1 ±0.5a | 17.2 ±0.3a | 13.4 ±0.5a | 1.28 ±0.11a |
| Group 1 | 35.9 ±0.2a, b | 30.3 ±0.7a, b | 19.3 ±0.6a, b | 1.57 ±0.15b |
| Group 2 | 37.2 ±0.5a, b | 31.8 ±0.6a, b | 20.1 ±0.8a, b | 1.58 ±0.17b |
| Group 3 | 39.5 ±0.4b | 35.1 ±0.2b | 22.7 ±0.2b | 1.55 ±0.19b |
| Group 4 | 38.1 ±0.3b | 35.7 ±0.7b | 22.5 ±0.5b | 1.59 ±0.16b |
In the group of control animals (untreated AA), there was a decrease in the number of total CD3+ T lymphocytes by 2.13 times, as well as subpopulations of CD4+ (helpers) by 2.12 times, CD8+ (suppressors) by 1.77 times and CD4+/CD8+ (IRI) by 1.2 times relative to the group of intact animals.
In animals with local administration of CrMMSCs from adipose and cartilage tissues (groups 1 and 2), the content of T cells was higher than the corresponding values in the control group (untreated AA). Namely, it respectively was 1.79 and 1.85 times for CD3+, 1.76 and 1.84 times for CD4+, 1.44 and 1.5 times for CD8+ and 1.22 and 1.23 times for CD4+/CD8+.
However, in groups 1 and 2, the content of T cells in the spleen did not reach the value in the group of intact animals. In the study of the subpopulation composition of spleen lymphocytes after cell therapy under generalized administration (groups 3 and 4), T cell content (CD3+, CD4+, CD8+, CD4+/CD8+) was normalized.
The next stage of the study was to investigate the content of CD4+CD25+ (Treg) in the spleen of animals with AA and therapy with CrMMSCs from adipose and cartilage tissues under local and generalized administration of the cells (Fig. 4).
Fig. 4
Effect of cryopreserved multipotent mesenchymal stromal cells from adipose and cartilage tissues on CD4+CD25+ cell content in spleen of rats with adjuvant arthritis (x ±SD, n = 5).
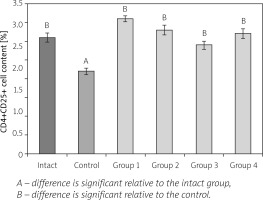
In the control group (untreated AA), there was a 1.41-fold decreased number of CD4+CD25+ cells relative to the group of intact animals. In the animals with local administration of CrMMSCs from adipose and cartilage tissues (groups 1 and 2), Treg cell content was respectively 1.66- and 1.51-fold higher than the corresponding values in the control group. In rats with generalized administration (groups 3 and 4), the studied index was 1.32- and 1.46-fold higher than the corresponding values in the group of untreated AA.
Discussion
We evaluated the effects of CrMMSCs transplantation on modulation of a few indices of cell immunity in adjuvant arthritis. It was found that AA, as an experimental analogue of RA, is a systemic pathology of connective tissue structures of the body.
After cell therapy, splenomegaly decreased; therefore, the corrective effect of CrMMSCs was pronounced in relation to the spleen index and cellularity and inhibition of hyperactive growth of connective tissue elements. It should be noted that in all experimental groups, the Treg content reached the intact group value.
At the same time, the increase in content of Treg cells in rats locally treated with CrMMSCs from adipose tissue was the largest among the all studied groups. Interestingly, the decreased level of Treg cells in the spleen of animals with AA is actually only a specific marker of pathology, since these cells do not have a protective potential, which contributes to the pathology manifestation in the organism.
The analysis of clinical results shows that in patients with RA there is a shift in the balance between auto-reactive T cells, which reflects a violation of mechanisms of pathological autoreactive suppression [2, 3, 9].
Therapeutic approaches aimed at restoring and normalizing the impaired function of immune systems under experimental AA can reduce the severity of inflammatory reactions and clinical manifestations of the disease.
Thus, our results confirm the occurrence of a large corrective effect on the main pathogenetic components of the immune system in animals that cause the AA manifestation. After CrMMSCs therapy, an increase in all studied lymphocyte subpopulations was noted in rats with AA that was accompanied by an approach to the control values of IRI.
T cells are activated during inflammation through several cell signaling processes. The initial signal that activates lymphocytes is mediated by antigen-specific T cell receptors, whereas the second phase, known as co-stimulation, is independent of these receptors yet critical for allowing complete immune response activation.
The multipotent mesenchymal stromal cells can suppress T cells at both the primary and secondary activation phases by signaling through soluble substances such as cytokines and growth factors, as well as through the mechanisms involving direct cell-to-cell interactions [18].
In our previous studies, we found that CrMMSCs could affect the reparative and regenerative processes in animals with both acute and chronic phases of inflammation [19, 20]. Local and generalized administration of CrMMSCs from adipose and cartilage tissues led to correction of the course of experimental AA in rats. The use of a local method of cell administration compared to the generalized one had a stronger effect on the restoration of histostructure, glycosaminoglycan content and COX-2 in cartilage tissue in the rats with AA.
The idea of our work was based on CrMMSCs therapy which would control the disease activity in AA rats not only through the anti-inflammatory and immune suppressive functions under generalized administration, but also through contribution to joint tissue restoration under local injection, and consequently averting cartilage damage.
In clinical practice MMSCs therapeutic methods for joint cartilage tissue repair have been tested in patients with arthritis/osteoarthritis with excellent results (involvement of MMSCs in tissue formation leading to cartilage repair) [21, 22].
So, the possible mechanisms through which MMSCs can affect the joint disease processes are varied and involve paracrine, anti-inflammatory, immune suppressive, and trophic effects. The significant decrease in the inflammation severity when using CrMMSCs from both studied sources for the animals with AA indicates the versatility of the mechanisms of their action in terms of the manifestation of an immune modulatory effect on the body, which leads to the normalization of immunity parameters.
However, an important issue that remains to be investigated is the determination of cellular immunity in the animals with AA and therapy of adipose and cartilage CrMMSCs with a combination of local and generalized administration.
Conclusions
Generalized and local administration of CrMMSCs derived from adipose and cartilage tissues led to a correction of the course of adjuvant arthritis which manifested itself in a decrease in the severity of clinical manifestations of the pathology and restoration of the number of regulatory cells in the spleen (CD4+, CD8+, CD4+CD25+).
The use of a generalized method of cell administration compared to a local one had a more powerful effect on restoring T cell content in rats with adjuvant arthritis. These data can be used to substantiate and develop methods of arthritis treatment in clinical practice.