Introduction
Each year, the number of joint replacement procedures performed increases. In the UK, hip replacement procedures have increased from 42,769 in 2004 to 82,824 in 2018 [1]. In our study, we wanted to find out whether physicians can prevent this state of affairs, as well as how factors other than a patient’s physical activity, diet, and lifestyle affect bone quality. As is well known, age also affects the condition of our musculoskeletal system. The aging process causes a decrease in bone mass and skeletal volume with varying degrees of severity depending on gender and ethnicity, leading to osteoporosis and an increased risk of fractures.
Cartilage wear, associated with age-related wear and tear of synovial joints, contributes to the development of osteoarthritis (OA), while the reduced structural integrity of intervertebral discs leads to loss of height, collapse, and compression in the spine [2].
In our research, we emphasize the impact of endocrine disorders on the skeletal system.
The complexity of the skeleton lies in its dependence on individual cell types such as osteocytes and chondrocytes, which function both remotely and in isolation. However, the proper functioning of these cell types depends on their smooth cooperation within larger systems [3].
Endocrine factors play an important role in regulating the interactions between cells affecting bone metabolism, mineralization and overall bone and muscle health.
The article aims to shed light on how disruptions in the endocrine system can negatively affect these delicate mechanisms, potentially leading to bone-related disorders and fractures. After analyzing the available literature, we created a paper showing how individual endocrine disorders affect the skeletal system and what should be done to prevent them.
Methods
Search strategy
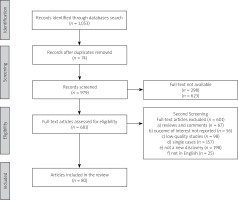
PubMed, Embase, Cochrane, and Web of Science databases were searched for related studies until 15 December 2022. A text search with the following key words singly or in combination was conducted: “Thyroid dysfunction”, “Obesity”, “Diabetes”, and “Musculoskeletal disorders” and duplicates were removed.
Results of searching are presented in Figure 1.
The role of thyroid hormones in bone metabolism
Studies conducted on a group of 5,458 people showed that in the population of people with hyperthyroidism compared to the general population, there was a greater annual bone loss at the femoral neck %ΔBMD = –0.18 (95% CI: from –0.34 to –0.02; I2 = 0%). Increased bone loss was noted especially in patients with thyroid-stimulating hormone (TSH) < 0.10 mIU/l [4].
Subsequent analyses (70,298 participants) showed that the age- and sex-adjusted hazard ratio (HR) for subclinical hyperthyroidism for hip fractures was 1.36 (95% CI: 1.13–1.64; 146 events in 2,082 participants vs. 2,534 in 56,471), and for vertebral fracture 1.51 (95% CI: 0.93–2.45; 17 events in 732 participants vs. 255 in 20,328). Lower TSH (0.10 mIU/l) was associated with higher fracture risk – HR 1.61 for hip fracture (95% CI: 1.21–2.15; 47 events per 510 participants), for vertebral fracture 3.57 (95% CI: 1.88–6.78; 8 events in 162 participants) [5].
These studies prove that thyroid disorders have a significant impact on the skeletal system.
Adequate action of thyroid hormones is necessary to maintain appropriate bone development, mineralization, and strength. Thyroxine and triiodothyronine work in two mechanisms, genomic and extragenomic, which result from interactions with extranuclear receptors. Inside the cell, these hormones interact with nuclear thyroid hormone receptors (TRs), which act as ligand-induced transcription factors to regulate triiodothyronine (T3) dependent gene expression [6]. TRα receptor is predominantly found in skeletal cells [7].
As population-based studies have shown, impaired bone remodeling and increased fracture risk in adults occur in both hyperthyroid and hypothyroid states. Thyroid pathologies are much less common in children. However, they can lead to disturbances in skeletal maturation, growth, and development of the skeleton in children. Furthermore, there is a proven correlation between subclinical hyperthyroidism, low bone mineral density, and an increased risk of fractures [8].
Receptors for the thyroid hormones TRα1 and TRβ1 are found in chondrocytes of the growth plate, bone marrow, stromal cells, osteoblasts, and osteoclasts, while their expression in osteocytes is in question. According to quantitative real time-PCR (RT-PCR) studies, TRα-1 levels are at least 10-fold higher than TRβ-1, indicating that TRα-1 is the main mediator of triiodothyronine (T3) action in bones [6].
Receptors for thyroid hormones compete with peroxisome proliferator-activated receptors (PPARs) for the retinoid X receptor (RXR). This is critical as PPAR-γ inhibits the synthesis of alkaline phosphatase (ALP) and type X collagen, and negatively affects TRα-1 transcription [7]. Thyroid-stimulating hormone receptor (TSHR) has been identified mainly in osteoblasts, osteoclast precursors, and osteosarcoma cells. Recently, a specific thyroid hormone transporter MCT8 has been reported to be expressed in the growth plate of chondrocytes, osteoclasts, and osteoblasts at all levels of differentiation. The expression of MCT10 and OATP1c1 in the skeleton has not been confirmed to date.
The levels of T3 and thyroxine (T4) hormones inside cells and their availability for receptors depend on 2 iodothyronine deiodinase (D2) and 3 iodothyronine deiodinase (D3) activity. Iodothyronine deiodinase 2 by removing the 5’-iodine atom converts T4 into T3. While D3 deiodinase deactivates T3. Iodothyronine deiodinase 2 has been detected in the embryonic mouse skeleton as early as the embryonic period, which may suggest that T3 plays an important role during fetal bone development. In the mature skeleton, T3 is found almost exclusively in osteoblasts and is activated by, among other factors, WSB-1 (PTHrP/Ihh intermediate feedback loop factor). Expression of D3 is widespread and the highest concentrations are observed in growth plate chondrocytes. Type 1 iodothyronine deiodinase (D1) is the only one among the deiodinases to catalyze the deiodinase reaction at the 5 ‘position (generation of T3 and T2) and the 5 positions (generation of rT3). The D1 enzyme is not found in bone tissue [7].
Triiodothyronine has been shown to stimulate alkaline phosphatase production and type II and X collagen. It is also responsible for regulating chondrocyte proliferation, stimulating differentiation, and angiogenesis, and inducing mineralization. In an animal model study, thyrotropin (TSH) was also found to block tumor necrosis factor α (TNF-α), which is one of the osteoclastogenic cytokines. TNF-α increases the differentiation of osteoclasts and thus plays an important role in the development of osteoporosis. Thus, TSH, by inhibiting TNF-α, has a protective effect on the development of osteoporosis.
The thyrotropic hormone also negatively affects osteoblast activity by reducing vascular endothelial growth factor expression. In osteoblast cultures, TSH stimulates D2, which contributes to increased levels of active thyroid hormones. Studies on TSH receptor-deficient mice support the hypothesis that thyrotropic hormone is a direct inhibitor of bone turnover, acting through TSHR in osteoblasts and osteoclasts. In addition to direct effects on chondrocytes via TR, thyroid hormones also exhibit indirect effects. They induce the production of insulin-like growth factor-1 (IGF-1), insulin-like growth factor-binding protein (IGFBP), interleukin-6 and -8 (IL-6, IL-8), fibroblast growth factor 1 receptor 1, prostaglandin E2, osteocalcin, osteopontin, and synthesis of metalloproteinase [9, 10].
Thyroid dysfunction
Hypothyroidism in childhood
The consequences of congenital and acquired hypothyroidism in young people are delayed skeletal development, growth inhibition, and impaired bone maturation [6]. Postmortem studies in hypothyroid rats showed a disorganized growth plate with abnormal matrix and angiogenesis [11].
Initiation of treatment results in a period of rapid growth, but the predicted growth and bone mineral density (BDM) can no longer always be achieved [6, 12]. In such cases, the magnitude of the growth deficit depends on the duration of untreated hypothyroidism and its severity [6]. Untreated patients may develop complete stunting and complex skeletal dysplasia. Hip dislocations, vertebral immaturity, scoliosis, delayed eruption of teeth, persistent fontanels, open cranial sutures, flat nasal ridges, and broad face are other characteristic features of ossification impairment [6].
Hyperthyroidism in childhood
Paradoxically, short stature is also a consequence of adolescent hyperthyroidism. This is the consequence of the initial acceleration of growth, premature bone maturation, and overgrowth of the growth plate [12]. Closing cranial sutures soon can also lead to craniosynostosis and neurological complications [7].
Hyperthyroidism at a young age can result from mutations in the receptor gene for TSH. Early treatment prevents the consequences of excess thyroid hormones [6]. Similar changes can occur as a result of TRβ mutation, leading to thyroid hormone resistance (RTH). The consequence is abnormally high levels of T3, T4, and TSH. Patients with RTH do not have a defined skeletal phenotype due to the presence of various mutations for TRβ and the effects of therapeutic intervention are difficult to predict [6]. The disease is inherited in an autosomal dominant manner [10].
Bone remodeling
The osteon, the structural unit of bone, is made up of bone lamellae arranged concentrically around the Haversian canal. The bone lamellae are formed by collagen, and between the lamellae, osteocytes are enclosed in so-called bone cavities. Inside the canal are a nerve, blood vessels, osteoblasts, and osteoclasts. Thyroid hormones play an important role in bone remodeling. Stimulation of osteoblasts is direct, while stimulation of osteoclasts occurs only in the presence of osteoblasts, this indicates an indirect effect through the action of cytokines [10].
Hyperthyroidism in adults
In hyperthyroidism, bone resorption and formation are accelerated and the remodeling cycle is shortened. The formation phase is shortened by approximately 30% and resorption by 60%. The frequency of initiation of bone remodeling is increased. Bone formation is increased to a lesser extent than bone resorption. This leads to a decrease in bone mass of approximately 10% per cycle [7].
These features also occur in patients with Graves-Basedow disease and toxic nodular goiter. Patients with hyperthyroidism have a negative calcium balance. This is caused by decreased absorption and increased loss of calcium in the urine, faeces, and skin. There is also a decrease in renal 1-alpha-hydroxylase activity, resulting in a decrease in calcitriol synthesis. This results in impaired absorption of calcium in the gastrointestinal tract which worsens the negative calcium balance. According to most studies, hyperthyroidism leads to a decrease in bone mineral density (BMD) of about 12–20% [9].
Untreated hyperthyroidism can lead to severe osteoporosis and pathological fractures [10]. There is little increase in BMD after treatment [9].
Thyroid dermopathy and acropachy
Thyroid dermatopathy and acropachy are rare complications of hyperthyroidism, especially in Graves-Basedow disease. The presence of thyroid dermatopathy and acropachy is a predictor of the severity of autoimmune processes.
Thyroid dermatopathy (pretibial edema) is caused by antibodies’ action on thyroid hormone receptors in the skin. It is estimated to occur in about 1–4% of patients with Graves-Basedow disease. It mainly affects the pretibial area. The skin becomes swollen, and red and resembles “orange peel” (peau d’orange) or “pig skin” [13–17].
Thyroid acropachy (or Palmar sign) mainly affects the fingers, toes and nose. It is estimated that about 20% of patients with thyroid dermatopathy also have thyroid acropachy. It is mainly characterized by redness, swelling and thickening of the skin. In severe cases, pain may also occur [13].
Treatment of both conditions is mainly based on the regulation of thyroid hormones and topical glucocorticosteroids [18, 19].
Hypothyroidism in adults
In hypothyroidism, the bone metabolism cycle is shortened. The duration of remodeling is prolonged. The resorption phase is prolonged 2 times, and the time of bone formation and secondary mineralization is prolonged 4 times. These changes result in an overall increase in bone mass and mineralization [7, 11]. The risk of fractures is increased. The hypothesis that this is influenced by the toxic effects of levothyroxine has been ruled out [7].
Patients with hypothyroidism have a slower metabolism, which can result in increased body weight, leading to the overloading of the osteoarticular system. Changes in the quality of bone structure also contribute to the increased incidence of fractures, reduced muscle strength, muscle cramps, pain and slowed movement, and rapid fatigability [8, 9].
Hypothyroid myopathy
Hypothyroid myopathy is caused by a deficiency of thyroid hormones. It can also occur in the subclinical form of hypothyroidism.
The pathogenesis of hypothyroidism myopathy is not fully understood. It is suggested that T4 deficiency leads to impaired glycogen breakdown in cells. As a result, type 2 muscle fibers atrophy occurs (since their main source of energy is glycolysis), and muscle contraction slows down. In addition, people with hypothyroidism experience a decrease in muscle carnitine, which contributes to myopathic symptoms. The compensatory response also results in muscle hypertrophy. This is due to an excessive accumulation of glycosaminoglycans in the muscles.
Symptoms of hypothyroid myopathy include poor muscle strength, muscle stiffness, difficulty moving and fatigue. Treatment mainly focuses on balancing thyroid hormone levels [20–22].
Euthyroidism
In numerous population-based studies conducted mainly in groups of postmenopausal women with normal thyroid hormone concentrations, a correlation between thyroid metabolic status and BDM has been proven.
The free thyroxine level was shown to be inversely correlated with BMD, while TSH levels were positively correlated – higher TSH levels were associated with higher bone mineral density (the association with fT4 was stronger). People with high normal thyroxine levels have an increased risk of osteoporosis, osteopenia, and non-vertebral fractures [7].
The effect of levothyroxine therapy on bone mineral density
The effect of levothyroxine treatment on bone mineral density is still unclear. Of the 63 studies, 31 showed no effect of levothyroxine on bone mineral density, and 9 reported general side effects. In 26, a partial beneficial effect was observed, however, it was accompanied by side effects. However, unfavorable results may be caused by the heterogeneity of these studies [11].
Obesity and bones
Obesity is a complex, multifactorial disease currently affecting one-third of the global population [23]. If trends continue, it is estimated that by 2030, 38% of the world’s adult population will be overweight and another 20% will be obese [24]. In the United States of America, the direct projections indicate that more than 85% of adults will be overweight or obese by 2030 [25]. According to the World Health Organization report from 2018, the prevalence of overweight (BMI value between 25 and 29.9), is 39% of the total population, of which 39% for men and 40% for women, and obesity (BMI of 30 or more) is 13% of the total population, of which 11% for men and 15% for women.
Around the world, the diet has been changing rapidly with less fresh vegetables and fruits and more calories from saturated fats and simple sugar in high caloric condensate foods. Moreover, lack of physical activity and chronic illnesses limiting mobility are major contributors to the development of overweight and obesity [26]. One of the most common predisposing factors is anxiety and eating disorders leading to increased consumption of food.
Obesity has a direct impact on health and increases the risk of many chronic diseases such as type 2 diabetes mellitus (T2DM), cardiovascular diseases, bone diseases, cancer, respiratory problems, and psychological problems which pose a great challenge for healthcare systems globally. All the complications have a significant impact on daily lives, reducing the quality of life and leading to premature death [27]. Obesity and overweight cause nearly three million deaths annually worldwide [24].
Obesity not only contributes to increased mortality but worsens quality of life and significantly weakens the skeletal system. A study of 44,793 patients with overweight and obesity found a significant link between obesity and musculoskeletal symptoms, with slower symptom recovery over one year of observation [28, 29].
Studies have also shown a higher risk of intervertebral disc degeneration in patients with overweight and increased body fat in those with sacroiliac and knee pain [30]. Osteoarthritis and sacroiliac pain occurred in 34% and 22% of obese patients, respectively [31]. Obesity is associated with changes in knee and foot posture and a higher risk of arthritis [32]. For obese patients requiring hip replacement, weight loss treatment is recommended before surgery [33]. Contrary to previous beliefs, obesity may increase the risk of fractures due to negative effects on bone structure, which is affected by factors such as glucocorticoids and pro-inflammatory cytokines [34, 35]. Obesity also increases the risk of OA and spinal pain syndromes [27, 36]. Weight reduction and regular physical activity can improve symptoms and quality of life. There is also a link between obesity and rheumatoid arthritis [36].
Factors affecting bone cells in obesity
Osteoblasts and adipocytes derive from mesenchymal precursor stem cells (MSCs). The balance between differentiation and proliferation of osteoblasts and adipocytes from one cell lineage is regulated by various factors [29, 37]. Obesity can lead to excessive adipogenesis, which may impair osteoblastogenesis. There is an interaction between adipose tissue and bone that is directly influenced by the hormonal function of adipose tissue. Obesity is associated with higher levels of circulating estrogens, which are the result of increased aromatization of androstenedione and testosterone by aromatase in white adipose tissue. In obese postmenopausal women, lower estrogen levels may partially increase bone loss as estrogen plays an important role in maintaining bone structure [30, 31].
Ghrelin is one of the hormones whose concentration increases in obesity. Ghrelin can potentially affect bone tissue as receptors for ghrelin have been identified on the surface of osteoblasts and may influence bone tissue and metabolism, but more research is needed [35].
Leptin, produced by white adipose tissue, plays a complex and controversial role in bone, affecting both bone formation and resorption [38, 39].
Insulin-like growth factor-1 (IGF-1) increases the differentiation and maturation of osteoblasts, playing a key role in bone growth [40]. Adipose tissue is a source of pro-inflammatory cytokines, such as TNF-α and IL-6, which stimulate osteoclast activity and bone loss, while reducing osteoblast differentiation. These cytokines affect the RANKL/RANK/OPG pathways, affecting osteoclast recruitment and bone resorption.
Obesity has complex effects on bone metabolism, involving various hormones and factors that affect the balance between osteoblasts and adipocytes. The exact interactions and implications for bone health require further study [40, 41].
Obesity, bone mineral density, and fractures
Until recently, it was thought that increased body weight led to higher bone mineral density (BDM) and reduced fracture risk in obese individuals [34]. However, recent evidence suggests that excessive body weight, especially fat accumulation, may increase fracture risk [35].
For postmenopausal women, obesity is associated with a higher risk of humerus and osteoporotic fractures of the ankle and lower limb, but a lower risk of hip, pelvis and wrist fractures. Data on men are limited but suggest similar patterns [34]. Some studies indicate a lower risk of proximal femur and spine fractures in obese adults, while abdominal obesity is associated with a higher risk of hip fractures [42, 43]. However, other studies reveal a negative association between obesity and fracture risk, with obese men at higher risk for fractures. Obese women have a lower risk of hip fractures, but a higher risk of ankle, tibia, humerus and vertebral fractures [44, 45].
In obese individuals, the force of the fall or the risk of the fall itself is higher, which may explain the higher incidence of fractures in areas of abundant cortical bone compared to normal-weight individuals. It is worth noting that differences in fat distribution also play a role in fracture susceptibility, with abdominal obesity increasing the risk of hip fractures [46].
Osteoarthritis
Osteoarthritis (OA) is characterized by pain and degenerative changes in the joints, as a result of abnormalities in the structure of the joints. There are many risk factors for the development of OA. The primary is related to gene expression and the secondary develops as a consequence of injury or diseases affecting the joints. The role of mechanical and metabolic factors in developing the disease in people with excessive body weight is emphasized. Inadequately low physical activity increases the likelihood of overweight, which in turn increases the chance of developing arthrosis. Also, inadequately high physical activity negatively affects joint strength.
The abnormal skeletal development has been involved in dysplasia of the hip, slipped capital femoral epiphysis, or Legg-Calve-Perthes disease, combined with overweight/obesity, which increases joint stress and exacerbates the symptoms of osteoarthritis. The key factors that increase the risk of gonarthrosis are visceral obesity and T2DM. In OA of the ankle or foot, mechanical factors play a key role, the increased load on the joints by increased body weight. In addition, impaired blood supply is also present and affects pain syndromes. As obesity progresses, the risk of developing OA increases [41].
Back pain syndromes in obese patients
The lumbar region is the most common location of pain among patients with obesity. The pain often radiates beyond the spine, referred to as sciatica. The pain response may impair bowel movements as well as urination as a result of the reflex response. The severity of symptoms is significantly greater among women. The influence of genetic factors has been excluded. Abnormal joint architecture in obesity most often results from joint degeneration. Discopathy begins to develop and may be exacerbated by obesity as early as in adolescence.
There are 3 stages of irreversibly progressive degenerative processes of deformation of the intervertebral discs: protrusion, extrusion, and sequestration. Cervical spinal pain syndrome manifests mainly as difficulty holding the head against the force of gravity. Symptoms are more pronounced in overweight patients because of low physical activity and reduced muscle mass and strength [42].
Diffuse idiopathic skeletal hyperostosis
Diffuse idiopathic skeletal hyperostosis (DISH) is characterized by a pathological process or osteophyte formation. They are located in the anterolateral part of the spine, especially in the anterior longitudinal ligament of the spine. The process affects especially the thoracic spine [43].
Diffuse idiopathic skeletal hyperostosis significantly more often develops among patients with obesity and T2DM as increased levels of adipokines and chronic inflammation are responsible for growth through the regulation of osteoblasts [44].
Diabetes and bone
Patients with both type of diabetes mellitus type 1 (T1DM) and T2DM have a significantly higher risk of bone fractures compared to people without diabetes. In a meta-analysis conducted among patients with T1DM and T2DM, the increased risk of any fracture, relative to those without diabetes, was proven as follows (relative risk [RR] 1.32, 95% CI: 1.17–1.48) [47]. Notably, T1DM significantly increased this risk of fractures, in comparison to T2DM, 1.51, CI: 1.35–1.68 vs. 1.22, CI: 1.13–1.31, respectively [47]. Moreover, a meta-analysis of the studies of the influence of T1D on the risk of osteoporosis revealed the overall risk of fracture was 3.16 (95% CI: 1.51–6.63; p = 0.002). In contrast, the risk of hip fracture was 3.78 (95% CI: 2.05–6.98; p < 0.001) and spine fracture 2.88 (95% CI: 1.71–4.82; p < 0.001) in comparison to the control group. In addition, T2DM also increases the overall relative risk of low-energy fractures in men and women by 137% and 87%, respectively [48].
The reason is not a difference in BMD, BMI, or falls. The cause is reduced resistance to bone fractures [49]. Diabetes reduces bone turnover by decreasing the receptor activator of nuclear factor κB ligand (RANKL), resulting in the inhibition of osteoclast recruitment and bone resorption, and increases the production of the Wnt inhibitor (Wnt) – sclerostin, which inhibits bone formation [50].
Bone marrow adipose tissue (BMAT) is found in both types of diabetes. It negatively affects bone by reducing osteogenic differentiation in favor of adipogenic differentiation. It also increases the pro-inflammatory state. RANKL is mainly produced by BMAT, which enhances osteoclastogenesis [50].
Skeletal homeostasis involves insulin sensitivity via peroxisome proliferator-activated nuclear receptor (PPAR) γ. The same posttranslational modifications of the PPARγ protein that regulate insulin sensitivity and energy metabolism are responsible for regulating bone turnover [51, 52]. Therefore, PPARγ can control the differentiation of cellular components of bone remodeling [52].
Mesenchymal stem cells (MSCs) can differentiate into adipocytes or osteoblasts depending on the pathway stimulation. In positive energy balance states pools of MSCs are scarce for osteogenesis since increased adipogenesis [53, 54]. High glycemic levels have been shown to decrease osteoclast autophagy, consequently increasing osteoclastogenesis [54]. Chronic hyperglycemia disrupts the bone marrow microenvironment, through abnormal angiogenesis and the development of vascular calcification. As a consequence, bone remodeling activity is reduced, followed by decreased bone quality and prolonged fracture healing time [55].
Insulin mechanism of in vitro action
Expression of the receptors for insulin is essential for the differentiation of the cellular pathway from pre-osteoblasts to mature osteoblasts [56]. Physiological concentrations of insulin ensure normal intracellular transport of glucose to osteoblasts and osteocytes via type 4 glucose transporters [57, 58].
Insulin also reduces osteoclasts’ activity. Osteoclastogenesis was found to be inhibited under hyperinsulinemic conditions, suggesting impaired bone metabolism in diabetic patients. A likely explanation for this phenomenon is the presence of significantly more insulin receptors on the plasma membrane of osteoclasts compared to osteoblasts [59].
Type 1 diabetes mellitus
Patients with T1DM have twice the risk of any fracture and four to five times the risk of hip fracture compared to those without diabetes [52]. There is already an increased risk of fractures in T1DM during childhood, before peak bone mass is reached, and continues throughout life [55]. Type 1 diabetes is characterized by low bone mineral density (BMD) that accounts for some, but not all, of the increased fracture risk [60]. Abnormalities in bone remodeling may result from the lack of anabolic effects of insulin on bone formation and changes in the growth hormone/IGF-I axis as a result of poor metabolic control [60, 61].
Proper treatment of hypoinsulinemia in patients with T1DM allows for increased bone mass [62]. In T1DM, exogenous insulin dose and residual C-peptide production have been shown to positively correlate with osteocalcin levels, a marker of bone formation [63]. Inadequate glycemic control, which may result from inadequate insulin doses and delivery, may result in low BMD in patients with T1DM. Mauriac syndrome is diagnosed in young patients with poor metabolic control of T1DM [59]. Low stature in patients suffering from Mauriac syndrome probably results from a deficiency of insulin-like growth factor-1 (IGF-1) or growth hormone [64].
Type 2 diabetes mellitus
Type 2 diabetes mellitus imposes an increased risk of fractures despite increased bone density, but the risk is lower than in T1DM [49]. Patients with diabetes have higher mortality from bone fractures and renal and cardiovascular complications. Bone fragility in patients with T2DM is associated with insulin resistance, reduced bone strength, abnormal mineralization, and predominance of biochemical markers of bone breakdown over bone formation [65, 66].
The enhanced synthesis of AGEs stimulates the production of IL-6 which inhibits the proliferation of osteoblasts and decreases their activity, increasing osteoclastic activity [65]. The AGEs bind to their receptor (RAGE) and activate the transcription factor – nuclear factor-κB (NF-κB). This results in increased expression of RANKL during osteoclastogenesis, and decreased differentiation of mesenchymal cells into osteoblasts [67].
Patients with T2DM have hypersecretion of calcium and decreased calcium absorption due to vitamin D deficiency. Furthermore, vitamin D deficiency also decreases osteocalcin secretion by osteoblasts resulting in decreased bone formation [68].
Musculoskeletal complications of diabetes
Carpal and ulnar tunnel syndromes
Carpal tunnel syndrome (CTS) is a common condition characterized by compression of the median nerve at the wrist and is commonly seen in the general population as well as in people with T1DM and T2DM [69]. Carpal tunnel syndrome is often considered part of the diabetic hand, a condition that encompasses the various hand-related complications seen in diabetes. In addition to CTS, the diabetic hand can include limited joint mobility (hand stiffenes), Dupuytren’s disease with contracture and flexor tendon sheath inflammation (commonly known as trigger finger). Moreover, ulnar nerve compression at the elbow is also considered part of this condition [70].
Reduced nerve flexibility has been demonstrated in patients with CTS compared to healthy individuals. Similarly, decreased nerve flexibility has been linked to increased neuropathy, particularly in peripheral nerves, in people with T2DM. Biomechanical factors, oxidative stress and microvascular abnormalities are also observed in diabetic neuropathy and contribute to various complications. Reduced elasticity of peripheral nerves can be attributed to impaired interlobular gliding due to biomechanical changes associated with neuropathy [69].
Adhesive capsulitis (frozen shoulder)
Adhesive capsulitis, also known as frozen shoulder. Patients with frozen shoulder often report a gradual onset of pain and progressive loss of range of motion in the glenohumeral joint without any specific injury. The limited range of motion follows a purse-string pattern, with external rotation being the most affected, followed by inversion in the scapular plane and then flexion. This pattern of restriction is characteristic of a frozen shoulder and helps distinguish it from other shoulder conditions [59, 69].
People with diabetes have a significantly higher risk of developing frozen shoulder compared to those without diabetes, with an estimated incidence of 13.4% among those with diabetes. The exact reasons for the link between diabetes and frozen shoulder are not fully understood. One hypothesis suggests that glycation processes in the body may lead to changes in the tissues of the shoulder joint capsule, contributing to the development of frozen shoulders. About 30% of people with frozen shoulder have diabetes, highlighting the strong link between the two conditions [71].
Tenosynovitis
Tendon sheath inflammation is a collective term for a group of inflammatory conditions that affect the synovial sheath surrounding tendons. It is characterized by inflammation, pain and limited mobility of the affected tendon and surrounding area. The incidence of stenosing tenosynovitis typically ranges from 1.7% to 2.6% in the general population. However, in people with diabetes, the incidence is much higher, ranging from 10% to 20%. Regardless of the cause, the mechanism involves inflammation within the tendon sheath [71].
Restricted joint mobility
In diabetes, the glycosylation process affects the structure of joints, ligaments and tendons, leading to impaired joint mobility and muscle function [72]. Foot disorders are a significant problem in diabetic polyneuropathy (DPN), as they can lead to ulceration and amputation. The progression of DPN affects small joints and intrinsic muscles, contributing to deformities, increased plantar pressure and ulceration. These changes affect the dynamic stability of the foot, leading to impaired mobility in daily activities. In addition, DPN is associated with difficulty in performing daily physical activities, altered gait biomechanics and a higher risk of falls [73].
A summary of the effects of thyroid dysfunction, obesity and diabetes on the skeletal system is presented in Table I.
Table I
The summary of the effects of thyroid disorders, obesity, and diabetes on the musculoskeletal system
| Thyroid action | Mechanism of action | Thyroid disorders-related changes in bones |
|---|---|---|
| The resorption phase is prolonged 2 times and the time of bone formation and secondary mineralization is prolonged 4 times The consequence of these changes is an overall increase in bone mass and mineralization | Hypothyroidism [7, 9] | An overload of the osteoarticular system and pathological fractures |
| The formation phase is shortened by approximately 30% and resorption by 60%; this leads to a decrease in bone mineral density (BMD) of about 12–20% | Hyperthyroidism [5, 7, 8] | Untreated hyperthyroidism can lead to severe osteoporosis and pathological fractures |
| Obesity | Mechanism of action | Obesity-related changes in bones |
| Increased levels of adipokines and chronic inflammation enhance processes of growth and regulation of osteoblasts. Insulin and growth hormone (IGF-1) play a key role in complex bone formation pathways | Hyperostosis [40, 41, 42] | Diffuse idiopathic skeletal hyperostosis (DISH) is characterized by a pathological process or osteophyte formation |
| An abnormal joint structure: 1) primary (idiopathic) osteoarthritis dependent on gene expression 2) secondary, depends on other factors (i.e. an injury) or disease affecting the joints | Osteoarthrosis [51] | Osteoarthrosis leads to pain and degenerative changes |
| Diabetes | Mechanism of action | Diabetes-related changes in bones |
| Type 1 diabetes (T1DM) is characterized by low bone mineral density (BMD). Abnormalities in bone remodeling may result from the lack of anabolic effects of insulin on bone formation and changes in the growth hormone–IGF-1 axis as a result of poor metabolic control. Hyperglycemia exacerbates the formation of advanced glycation end products (AGEs). The accumulation of AGEs in the skeleton leads to a decrease in bone strength, increasing susceptibility to fracture | T1DM and fracture [66, 75, 76, 86, 87] | An increased risk of fractures |
| Bone fragility in patients with type 2 diabetes (T2DM) is associated with decreased bone strength, defects in collagen fibres resulting in abnormal mineralization, and increased bone microdamage | T2DM and fracture [54] | An increased risk of fractures |
| Poor metabolic control of T1DM can cause glycogenic degeneration of the liver (hepatopathy) and may be associated with hypercholesterolemia and a shortage of IGF-1 and growth hormone | Mauriac syndrome [56, 57] | Mauriac’s syndrome is characterized by low stature, a cushingoid appearance, and delayed puberty |
Treatment of type 2 diabetes and its influence on bone metabolism
Insulin
At very low concentrations of insulin, the quality of bone expressed as skeletal microarchitecture is impaired, due to a decrease in the intensity of bone formation or a reduction of the RUNX2 factor (a marker of osteogenesis). Insulin supply improves bone microarchitecture [74]. The latest data revealed that patients with T2DM using insulin therapy have an increased risk of fractures [60].
Insulin therapy may have direct negative consequences with an increased risk of hypoglycemia and an increased incidence of falls, the presence of renal complications. The need for insulin treatment in patients with T2D also indicates a more advanced stage of the disease which may also influence increased fracture rates [52].
Thiazolidinediones
Thiazolidinediones (TZDs) slow down the development of diabetes and improve patients’ glycemic control. However, adverse effects such as weight gain, congestive heart failure, bone fractures, and possibly bladder cancer have been noted while using TZDs. Thiazolidinediones has been proven to increase the risk of fractures in women, especially in advanced-age or postmenopausal women [75].
The use of TZD in the elderly results in an increase in the number of osteoclasts and increased bone resorption, probably due to the drug sensitization of osteoclast precursors to other factors stimulating osteoclastogenesis [75, 76]. Thiazolidinediones also reduce circulating insulin levels, which inhibits its anabolic effect on osteoblasts. As a result, apoptosis of osteoblasts and osteocytes is promoted. Also, impaired bone formation leads to a reduction in the volume of trabecular bone and BMD [67]. The activation of PPAR inhibits bone formation, primarily by redirecting mesenchymal stem cells to the adipocytic rather than osteogenic lineage increasing bone resorption by stimulating osteoclasts [77].
The concentration of rosiglitazone varies between individuals due to genetic variations in CYP2C8*3 activity, an enzyme implicating in the metabolism of rosiglitazone and thus different therapeutic responses may be observed in patients with a different effect on bone turnover [67, 78].
Biguanidines
Metformin enables glycemic control by positively affecting the sensitivity of cells to insulin. It also improves bone quality and reduces the risk of fractures in diabetic patients. It has a low risk of hypoglycemia and there is no weight gain. Metformin enhances osteogenesis in vivo as well as in vitro and has beneficial effects on bone repair processes in non-T2DM animals. Metformin may increase the maturation and differentiation of osteoblasts through the induction of endothelial nitric oxide synthase (eNOS) [79].
The AMP-activated protein kinase signaling pathway has beneficial effects on bone metabolism by stimulating osteogenesis, and inhibiting adipogenesis in bone (MSCs) through the Runx2 and Wnt/β-catenin pathways [80].
Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) regulates glucose through insulin secretion, decreases glucagon secretion, and enhances osteogenesis. The effect of GLP-1 receptor agonists on bone is not fully understood, and its use in the prevention of osteoporosis and fractures is controversial [81]. In a study by Gilbert et al. [82], the results showed that liraglutide therapy in patients with T2DM did not increase total bone mineral density and the results were similar in other studies [81, 82].
DPP-4 inhibitors
DPP-4 inhibitors (DPP-4i) increase GLP-1 concentrations, increase insulin secretion and inhibit glucagon secretion which results in improved carbohydrate metabolism in patients with T2DM [83]. The results of a study by Hegazy [84] revealed that the use of sitagliptin in patients with diabetic osteoporosis resulted in a significant reduction in urinary deoxypyridinoline secretion. It was hypothesized that DPP-4i influences bone metabolism by reducing resorption rather than its formation [84].
Furthermore, the results of a study by Chailurkit et al. [85] showed that the use of DPP-4i resulted in the stimulation of osteoblasts, the reduction of advanced glycation end products, and bone resorption.
Sodium-dependent glucose co-transporter type 2 inhibitors
Sodium-dependent glucose co-transporter type 2 (SGLT-2) inhibitors (SGLT-2Is) present a neutral effect on bone metabolism and do not increase the risk of fractures [50].
Conclusions
Thyroid dysfunction, obesity and diabetes affect the function of the skeletal system. Endocrine disorders should be considered and diagnosed before surgery procedures therefore appropriate treatment may change the prognosis and effectiveness of overall management.
As our population ages, obesity and metabolic syndrome and diabetes rates increase, it has become crucial to understand the interplay between obesity, diabetes, hypothyroidism and its consequences on musculoskeletal system. This understanding is essential to reduce the societal and individual costs associated with osteoarthritis, osteoporosis and osteoporotic fractures.