Introduction
Diabetes mellitus (DM) and osteoporosis (OP) are very common diseases and their frequency is increasing in the aging population. In recent years weak bone quality and increased fracture risk associated with diabetes have been reported [1–3].
It is known that the risk of fractures due to diabetes is significantly higher and often is not reflected in bone mineral density (BMD) as measured by dual energy X-ray absorptiometry (DXA). As a result, other BMD-independent diagnostic measures, including trabecular bone score (TBS) assessment, have been introduced into the risk assessment strategy [4].
The aims of our review are to present the relationship between the occurrence of OP and DM and discuss differences between the pathogenesis of OP depending on types of DM. We also propose an effective diagnostic strategy for identifying OP and fracture risk assessment in diabetic patients.
Methods
Databases such as MEDLINE, COCHRANE and SCOPUS were analyzed to search for reports, reviews, and meta-analyses published in the period 2016–2022 and present diagnostic methods, fracture risk assessment, prevention of OP and fractures in DM patients as well as treatment of osteoporosis and osteopenia in this patient group.
The key words for the search were: diabetes, osteoporosis, and low-energy fracture.
Diagnosis of osteoporosis and fracture risk assessment
It is estimated that more than 200 million people in the world have osteoporosis and nearly 9 million osteoporotic fractures occur annually [5]. The World Health Organization (WHO) defines osteoporosis as a T-score of –2.5 or less based on BMD measurement, or based on the occurrence of a low-energy fracture in people with osteopenia. However, it is known that the number of factors influencing the risk of fracture is greater than that resulting from the BMD values [6].
The International Osteoporosis Foundation (IOF) and WHO recommend the FRAX calculator (Fracture Risk Assessment Tool), which takes into account not only BMD of the femoral neck, but also other risk factors, such as age, sex, weight, height, previous low-energy fractures, parental hip fracture, smoking, use of glucocorticosteroids, rheumatoid arthritis, secondary osteoporosis, and alcohol consumption above 3 units per day, to estimate the 10-year probability of a major osteoporotic fracture in people aged 40 to 90 years [7]. According to the FRAX calculator, only type I (insulin dependent) diabetes is recognized as a secondary cause of osteoporosis. Therefore, probability calculated by FRAX may underestimate fracture risk in type 2 diabetes [8].
Diabetes
Diabetes mellitus is a global problem, and the number of diabetic patients is constantly increasing. The most common form – diabetes type 2 (T2DM) – is caused by insulin resistance leading gradually to progressive impairment of insulin secretion. In turn, chronic disorders of glucose metabolism are connected with a number of symptoms and complications, including increased cardiovascular risk, microangiopathy, cataracts, susceptibility to infection and an increased risk of low-energy fractures [9]. Densitometrically confirmed OP is the most significant metabolic bone disease in patients with type 1 diabetes (T1DM), while low-traumatic fractures occur in both types of diabetes [10].
The pathomechanisms leading to impaired bone health in diabetes are unknown. Type 1 and 2 diabetes mellitus were connected with a general increased fracture risk, with preferential fracture sites at the hip and vertebrae, and likely the humerus, distal forearm, and foot in T2DM. In T1DM the most frequent fractures are the hip, vertebrae, humerus, and ankle [11]. High fasting glucose variability is associated with higher risk of hip fracture [12].
Sarcopenia, obesity and other diabetic disorders such as cognitive impairment, peripheral neuropathy, visual impairment, cardiac arrhythmias, and hypoglycemic episodes increase the risk of falls and fractures [13]. Other skeletal consequences of chronic hyperglycemia include hypercalciuria caused by glycosuria, and different, not quite clear interactions of high glucose levels with the parathormone and vitamin D [14]. Furthermore, many patients with T2DM have low serum 25(OH)D3 concentrations due to low physical activity, obesity and less sun exposure [15]. Common risk factors such as smoking, osteoporotic fractures in parents, risk of falls etc. should be evaluated as well.
Considering all these factors, the early estimation of fracture risk and diagnosis of metabolic bone diseases in diabetic patients are essential.
Bone complications of T1DM are more severe than T2DM, because of the lack of anabolic effect of insulin on bones [16, 17]. In children and adolescents the lack of insulin and low IGF1 levels inhibit the terminal differentiation of osteoblasts from mesenchymal stem cells, which leads to low peak bone mass [18]. There is a considerable risk of comorbidity of T1DM with other auto-immune diseases such as rheumatoid arthritis, celiac disease and for example autoimmune thyroid diseases, which also increase the risk of fractures [19].
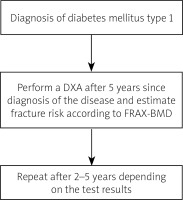
In adult patients with T1DM, the first densitometry should be performed five years after the diagnosis of the diabetes mellitus and repeated every 2–5 years. The FRAX tool is not appropriate for assessing the fracture risk in young patients with T1DM. It is quite useful in older patients with T2DM. However in these patients the calculated fracture risk may be underestimated [20].
Similarly in T2DM the fracture risk often does not correspond to the BMD value as measured by DXA. However, taking into account the conducted studies, it was found that these patients tend to have low-energy fractures with higher BMD values compared to the healthy population [21]. Moreover, the incidence of fractures in patients with osteopenia is significantly higher in diabetic patients than in healthy subjects [22].
It has been proven that patients with T2DM have a higher risk of fracture than the healthy population at the same BMD value [23], which may be connected to deterioration of bone quality and bone microarchitecture disorders [24, 25]. Some experts do not call this osteoporosis but diabetic bone disease [26]. In Table I the differences and similarities between T2DM and T2DM are presented.
Table I
Differences and similarities in T1DM and T2DM
Trabecular bone score
Importantly, bone strength and fracture risk are affected by factors other than BMD. Microarchitectural deterioration of bone tissue, structural micro-damage, and bone turnover and a wide range of clinical risk factors such as age, prior fracture, family history and fall risk contribute to the general assessment of fracture risk [27].
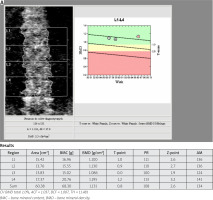
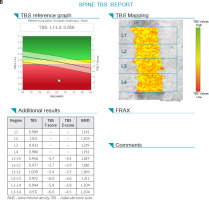
The trabecular bone score (TBS) is an index, derived from DXA scans of the lumbar spine, which facilitates the analysis of bone microarchitecture evaluating trabecular number, density and separation [28]. It allows vertebral fracture risk assessment independently of BMD. It has been shown that TBS predicts fractures regardless of the fracture probability assessed with the FRAX calculator. Clinical trials have shown that TBS is important in the assessment of fracture risk in other causes of secondary osteoporosis for example hyperparathyroidism and glucocorticosteroid-induced osteoporosis [29, 30].
An elevated TBS means fracture-resistant and strong bone microarchitecture. A low TBS reflects weaker, fracture-prone microarchitecture of bone. The largest published study assessing TBS in 29,407 postmenopausal women over 50 years old in the Canadian province of Manitoba revealed that lumbar spine BMD and TBS predicted fractures equally well [31]. Moreover the combination of both anticipated fractures better than either individually [32].
The Manitoba data were used to derive an adjustment factor to change the fracture risk probability calculated by FRAX [33]. Also it is worth emphasizing that in the Ansung cohort (1,229 men and 1,529 postmenopausal women including 325 men and 370 women with type-2 diabetes) lumbar spine TBS was lower in both sex with diabetes than in healthy patients, but lumbar spine BMD was higher in men and women with diabetes. The trabecular bone score negatively correlated with HbA1c (hemoglobin A1c) and fasting plasma glucose levels [34, 35]. It seems that TBS is an important additional factor supporting fracture risk assessment in patients with T2DM.
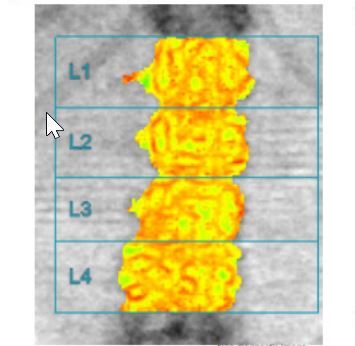
In Figure 1 A and 1 B the picture of osteopenia in DEXA and serious deterioration of trabecular bone architecture in TBS assessment in one patient is presented. In Figure 2 A and 2 B the situation of a significant difference between the picture of DXA (normal) and trabecular bone damage in TBS (another patient) can be seen.
Fig. 1 A
Bone mineral density of lumbar spine in patient (68-year-old woman) with T2DM, which indicates osteopenia.
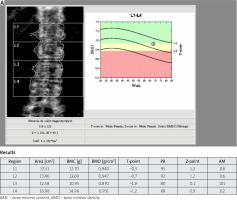
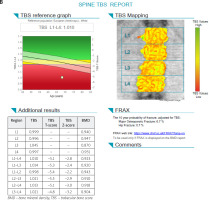
Fig. 1 B
Trabecular bone score of the same patient which indicates serious deterioration of trabecular bone microarchitecture.
Bone turnover and bone turnover markers
The extracellular bone matrix consists of inorganic minerals and organic structures. The inorganic component consists mainly of hydroxyapatite and is responsible for stiffness and mechanical resistance. The degree of tissue mineralization is measured by DXA and expressed by BMD. The organic component consists of collagen fibers and provides bone strength.
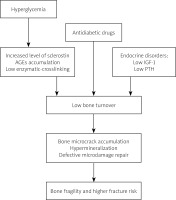
The pathogenesis of diabetic bone disease with deteriorating bone quality is multifactorial and not quite clear. Diabetes causes damage to bone microcirculation, impairs osteoblast maturation and bone formation, and affects bone turnover [36]. Decreased blood PTH levels, oxidative stress and chronic hyperglycemia lead to the deposition of advanced glycation end products (AGEs). Non-enzymatic metabolism of many proteins (including collagen) increases the concentration of sclerostin, an inhibitor of osteoblasts [37, 38]. In addition, obesity, low physical activity, some antidiabetic drugs such as thiazolidinediones, sodium-glucose cotransporter inhibitors [39] and a low level of insulin-like growth factor-1 (IGF-1) are associated with metabolic alterations in bones [40].
Metabolic disorders in the bones, both known and unknown, lead to hypermineralization, bone microcrack accumulation and defective microdamage repair [41]. Although bone mass is high in patients with T2DM, it does not protect against fractures because of impaired quality of bone tissue [42]. It has been found that changes in bone metabolism and damage to the microcirculation can increase fracture healing time by 87% [43].
In healthy individuals, the fracture response occurs within the first few weeks of recovery. It is expressed by peak levels of increased bone turnover markers such as osteocalcin, alkaline phosphatase (ALP) and IGF-1 [44]. Conversely, in diabetic patients bone turnover markers are reduced after a fracture [45], which is presumably associated with a slower healing process.
Bone metabolism can be measured indirectly using markers of bone turnover including s-procollagen type 1 amino terminal propeptide (P1NP), osteocalcin, C-terminal cross-linked telopeptide of type-I collagen (CTX) and sclerostin. In particular, the osteocalcin produced by osteoblasts and P1NP are markers of bone formation. Children with T1DM have low osteocalcin levels, which negatively correlate with diabetes control and HbA1c levels [46]. Osteocalcin serum levels are decreased in both types of diabetes compared to healthy people [47]. On the other hand, sclerostin as a marker of bone resorption, is negatively correlated with markers of bone formation in T2DM patients [48].
Studies assessing bone remodeling have shown decreased bone formation, with reduction in the mineralization. Moreover, CTX serum levels were also reduced [49]. Other markers, such as bone-specific alkaline phosphatase were within the normal range. Suppression of bone resorption may be additionally caused in T2DM by elevated blood levels of osteoprotegerin, which binds to RANKL (receptor activator of nuclear factor κB ligand) leading to inhibition of osteoclasts maturation. All these metabolic mechanisms do not lead to the development of typical osteoporosis, and for this reason it is instead referred to as a diabetic bone disease [50]. In Figure 3 the mechanism of diabetic bone disease is presented.
Drugs used to treat diabetes
Hyperglycemia control is the most important treatment strategy for diabetic bone disease.
Drugs used to treat T2DM can have both indirect and direct effects on bone metabolism and fracture risk. Observations so far show that some drugs have a beneficial effect on bones, but there are groups of drugs that should be avoided. Metformin (a biguanide anti-hyperglycemic drug), dipeptidyl peptidase-4 inhibitors, sulfonylureas and glucagon-like peptide-1 receptor agonists should be the first line treatment of diabetes in patients with osteoporosis [51].
Thiazolidinediones (TZDs) and canagliflozin should be avoided. The other SGLT-2 inhibitors are the less explored options. Thiazolidinediones are peroxisome proliferator – activated receptor γ agonists, which are no longer widely used. They could cause an increased risk of fractures because they induce increase osteoblast apoptosis and promote differentiation of multipotent mesenchymal stem cells into adipocytes [52].
Another group of drugs that have a negative effect on the bones is SGLT-2i. These drugs inhibit sodium-glucose cotransporters and their metabolic action consists in inhibiting the reabsorption of glucose in the kidneys, which leads to a decrease in glycemic levels. On the other hand, these drugs may disturb the level of calcium and phosphate metabolism, causing loss of bone mineral density (BMD) and more frequent falls. In terms of bone health, canagliflozin has been shown to have a negative effect on bone density, bone resorption, and the risk of hip fracture [53].
However, there are groups of antidiabetic drugs that have a beneficial effect on bone metabolism.
Metformin is the most commonly used oral hypoglycemic drug. By making tissues more sensitive to insulin, it improves glycemic control and may slow down the development of diabetic bone disease.
Sulfonylureas are among the commonly prescribed antidiabetic drugs. They are proved to be effective in controlling glycemia and preventing microvascular complications. Studies indicate a positive or at least neutral effect of sulfonylureas on fracture risk [54]. Hypoglycemia causing falls is a common side effect of these drugs, and therefore the risk of fractures may be increased [55].
Incretins are hormones that stimulate insulin production, inhibit the release of glucagon and slow down the rate of absorption of nutrients into the bloodstream by delaying gastric emptying. Incretin-based drugs used in the treatment of T2DM are glucagon-like peptide-1 receptor agonists and inhibitors of the enzyme dipeptidyl peptidase-4. To date, studies have shown that treatment with these drugs has a neutral or beneficial effect on the risk of fractures [56]. They may improve potentially positive effects on bone by calcitonin production in thyroid C-cells, which inhibits bone resorption [57].
Insulin (protein hormone) is the basic drug which replaces the endogenous hormone in the treatment of T1DM and supporting in severe forms of T2DM. Insulin should be used with caution to avoid hypoglycemia. In patients with T1DM before starting treatment, the incidence of OP or osteopenia was found to be significantly higher. It has been proven that after several years of taking insulin, bone turnover markers and BMD had significantly improved [58]. In Figure 4 the therapeutic management of osteoporosis in diabetes mellitus is presented.
Drugs used to treat osteoporosis
There is currently no evidence of the effect of anti-osteoporotic drugs on glucose metabolism. Therefore treatment of osteoporosis should not be modified due to the presence of T2DM and T1DM [59].
Additional risk factors
Clinical management should always focus on avoiding known risk factors, implementation of preventing falls strategies, maintaining good glycemic control, and making early diagnosis in people at risk of fractures. Other lifestyle changes such as supplementation appropriate dietary calcium and vitamin D, regular physical activity, avoidance of alcohol and smoking should be recommended. Obesity is typical for patients with T2DM and is itself connected with an increased risk of fractures at certain localizations such as tibia, humerus, and ankle [60]. Importantly, weight loss adversely affects the skeletal system by reducing bone mass. There are data that adipocytes which primarily compose adipose tissue arise from nonhematopoietic mesenchymal stem cells like osteoblasts [61].
Leptin, resistin and adiponectin affect on skeletal system. Resistin and adiponectin implicate beneficial effects of obesity on the skeleton. Leptin stimulates osteoclastogenesis inhibition and osteoblastic differentiation [62–64]. Clinical studies revealed that serum levels of leptin positively correlate with BMD [65].
To summarize the risk of fracture in T1DM is high. The general guidelines of managing osteoporosis in diabetes are early diagnosis and implementation of screening tests, prevention of hypoglycaemia and falls, propriate glycaemic control, suplementation of witamin D and exercise programs to increase muscle and bone strength. In patients with T1DM first densitometry should be performed earlier than 5 years after diagnosis of the disease (Fig. 5). Currently, there are no separate recommendations for diagnostic management in patients with T2DM, which requires further research.
Conclusions
In T2DM the risk of fractures is elevated, however, identifying the mechanisms underlying the increased risk of fractures in T2DM is not clear. Optimal strategies to identify and treat high risk individuals requires further research and proper definition. It is worth noting, that the fracture risk based on FRAX calculation may be underestimated and in T2DM may not match BMD values.
Therefore, bone health in diabetes need further investigations in search of the best methods for evaluating bone microarchitecture and alterations in bone quality. The diagnostic criteria for osteoporosis should be clearly defined as well as fracture risk assessment and choice of anti-osteoporotic medication. Diagnostic tools such as TBS may play an siginicant role in this group of patients. In all cases of secondary osteoporosis, treatment of the underlying disease is the most important. The relationship between high risk of fractures and diabetes is inseparable, and its full understanding seems to be the key to effective management.