Introduction
Ankylosing spondylitis (AS) is a common rheumatic disease characterized by polymorphism of clinical manifestations and difficulty in diagnosis, especially in the early stages of the process [1]. One of the key signs of AS is pathological bone formation, which leads to ankylosis of the spine and sacroiliac joints [2, 3]. On the other hand, AS is characterized by progressive bone mass loss and the development of osteoporosis [4, 5]. Opposite pathological processes of ossification and bone mass loss can occur simultaneously and close in the spine, thus leading to structural damage and pronounced functional disorders [6]. In particular, syndesmophytes can lead to reduced spinal mobility, and systemic bone tissue loss can cause vertebral fractures [7]. In addition, the chronic inflammatory process can indicate increasing bone mineral density (BMD) in the later stages of the disease due to development of syndesmophytes and bony bridges. However, real bone density was better assessed with the trabecular bone score (TBS) [8]. To date, the question of what leads to bone mass loss and bone tissue formation, and whether these processes are related to inflammation, remains poorly understood. In previous studies, it was reported that at the beginning of the disease, a decrease in BMD still prevails over osteoproliferative changes [9] and is associated not only with a number of general factors (age, sex, smoking, low body weight, hypogonadism) but also with the direct influence of a persistent inflammatory process, long-term use of glucocorticsteroids (GCs), vitamin D deficiency, decreased motor activity, etc. [3, 7, 10]. The absolute and relative contribution of each of these factors has not been established with certainty. Spinal fracture is an important complication of AS, the prevalence of which varies from 1 to 40%, which is more than four times higher than in the general population [4, 11, 12]. In the Ukrainian population of patients with AS, we did not find data on the frequency of osteoporosis, osteopenic syndrome, and low-energy fractures. The influence of the course of the disease on the formation of BMD disorders also remains unstudied.
Therefore, the aim of the research was to study the structural and functional state of bone tissue in men with AS and to assess its relationship with the course of the disease.
Material and methods
In a randomized manner with preliminary stratification according to the presence of AS, diagnosed according to the modified New York 1984 criteria, ASAS criteria, 105 patients (100% men) aged 22 to 59 years (average age was 40.7 ±0.8 years) with a disease duration of 8.7 ±0.5 years, who were treated in the rheumatology department of the Scientific Research Institute for the Rehabilitation of Persons with Disabilities (educational and scientific medical complex) of Vinnytsya National Pirogov Memorial Medical University and did not receive any medications for the treatment of osteoporosis, took part in the study after signing the voluntary consent to participate. The control group included 29 men who did not have any rheumatological pathology at the time of examination or in the anamnestic history. All patients underwent a comprehensive clinical and laboratory examination. The determination of clinical activity of AS was based on the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Activity Score correlated with C-reactive protein (ASDAS-CRP) (< 1.3 – inactive AS; 1.3–2.1 – moderate activity; 2.1–3.5 – high activity; > 3.5 – very high activity), and functional capacity was assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI). The level of the inflammatory activity marker CRP was analyzed using standard laboratory methods in a medical institution.
Bone mineral density of the lumbar spine and femoral neck was determined using the method of the dual-energy X-ray absorptiometry (DEXA) on the Hologic Discovery W device (S/N 87227). The diagnosis of osteoporosis in patients aged above 50 years was considered in the case of a decrease in BMD according to the T-score ≤ –2.5 SD, while osteopenia corresponded to T-score values ranging from –1 to –2.5 SD. For men aged up to 50 years, the Z-score was used; its value below ≤ –2.0 SD or less indicated a significant loss of bone mass. In order to determine the presence of osteoproliferative changes and the development of osteophytes, the patients underwent an X-ray examination of the thoracic and lumbar regions of the spine. In addition, deformations and fractures of the vertebral bodies were determined on standard X-rays of the thoracic and lumbar sections of the spine.
Statistical analysis
Statistical processing of the obtained results was carried out using Microsoft Office Excel for Windows 2007 and the statistical program Statistica SPSS 10.0 for Windows. The following statistical values were determined: number of observations (n), arithmetic mean (M), standard error of the average value (m), and absolute and relative values (abs., %). The normality of the distribution was checked by the Shapiro-Wilk test. The parametric Student’s t-test was used to assess the difference between groups for a normal distribution, and the Mann-Whitney U test for a distribution deviating from the normal distribution. When comparing the frequency of changes, Fisher’s exact method was used, and the correlation of characteristics between indicators was determined using Pearson’s correlation analysis (r) (differences at p < 0.05 were considered significant).
Bioethical standards
As a result of the examination carried out by the bioethics committee of Vinnytsya National Pirogov Memorial Medical University (No. 86/12 of 2023), it was established that the research methods do not contradict the basic bioethical norms of the Declaration of Helsinki and correspond to human rights under the current legislation of Ukraine.
Results
In men with AS, a decrease in BMD at the level of the lumbar spine and femoral neck was observed in 44 people (41.9%). At the same time, in the age group up to 50 years, low BMD, determined by the Z-score, was found in 29 (33.3%) people, which is 4 times higher than in the control group – 2 (8.3%). The average level of the Z-score among individuals of the control group at the level of the lumbar and femoral neck was 0.12 ±0.2 and –0.13 ±0.1, and in AS patients, it was significantly lower and equal to –1.2 ±0.2 and –0.9 ±0.1, respectively. In the age group over 50, the proportion of patients with osteoporosis at the level of the lumbar spine and femoral neck was 3 (16.7%), which is significantly lower than in the control group, and the osteopenic syndrome was detected in almost every second patient with AS, and at the level of the femoral neck in 66.6% of people. Additional confirmation of low bone tissue density in men with AS is the determination of the BMD level in both studied areas. The average values of BMD at the level of the lumbar spine did not differ significantly between the control and main groups, and at the level of the femoral neck they were 16.6% lower in AS patients under the age of 50 (0.75 ±0.01 g/cm2) and 30% lower in the group of patients over 50 years old (0.74 ±0.01 g/cm2) compared to the control group (0.9 ±0.02 g/cm2 and 1.06 ±0.02 g/cm2). The frequency of osteoporotic fractures among AS patients was 11.4%, while in the control group it was 3.5%. BMD disorders in men with AS were also associated with osteoproliferative changes. In particular, the percentage of patients with syndesmophytes was 40% (Table I).
Table I
Structural and functional characteristics of bone tissue in men suffering from AS and individuals of the control group
| Characteristics | Zone impression | Groups of patients | |
|---|---|---|---|
| Control group | Patients with AS | ||
| Men under 50 years of age | n = 24 | n = 87 | |
| Average value of BMD [g/cm2] | Lumbar spine | 1.07 ±0.02 | 0.93 ±0.02 |
| Femoral neck | 0.9 ±0.02 | 0.75 ±0.01 | |
| Z-score (M ±m – standard deviation in relation to the general population of a given age) | Lumbar spine | 0.12 ±0.24 | –1.2 ±0.2* |
| Femoral neck | 0.2 ±0.14 | –0.9 ±0.1* | |
| Patients with low BMD (Z-score ≤ –2.0) | Lumbar spine | 2 (8.3%) | 29 (33.3%)* |
| Femoral neck | 2 (8.3%) | 6 (6.9%) | |
| Patients with preserved BMD (Z-score > –2.0) | Lumbar spine | 22 (91.6%) | 58 (66.7%)* |
| Femoral neck | 22 (91.6%) | 81 (93.1%) | |
| Men over 50 years old | n = 5 | n = 18 | |
| Average value of BMD [g/cm2] | Lumbar spine | 0.99 ±0.03 | 1.00 ±0.01 |
| Femoral neck | 1.06 ±0.02 | 0.74 ±0.01* | |
| T-score (M ±m) | Lumbar spine | –0.24 ±0.2 | –0.8 ±0.4 |
| Femoral neck | 0.82 ±0.1 | –1.5 ±0.2* | |
| Patients with osteoporosis T-score ≤ –2.5 | Lumbar spine | 1 (20.0 %) | 3 (16.7%) |
| Femoral neck | 1 (20.0%) | 3 (16.7%)* | |
| Patients with osteopenia T-score from –1.0 to –2.5 | Lumbar spine | 2 (40.0%) | 8 (44.4%) |
| Femoral neck | 2 (40.0%) | 12 (66.6%) | |
| Patients with preserved BMD T-score > –1.0 | Lumbar spine | 2 (40.0%) | 7 (38.9%) |
| Femoral neck | 2 (40.0%) | 3 (16.7%) | |
| Total number of people with low BMD, n = 29/105 | 5 (17.2%) | 44 (41.9%)* | |
| Total number of people with fractures, n = 29/105 | 1 (3.5%) | 12 (11.4%) | |
| Number of people with syndesmophytes, n = 29/105 | – | 42 (40.0%) | |
When analyzing the age characteristics, we found that BMD indicators in both studied zones differed as the age of the patients increased. In particular, the average values of Z-score, T-score and BMD index at the level of the lumbar spine were the lowest in the age group of 18–29 years (–1.55 ±0.2; –1.56 ±0.2 and 0.91 ±0.02 g/cm2), while in the age category of patients 45–59 years, the indicators significantly increased to the level of –0.3 ± 0.4; 0.8 ±0.4 and 1.00 ±0.01 g/cm2. At the level of the femoral neck, the average values of the Z- and T-score were not related to the age of the patients, and the BMD index was practically the same among the studied groups. The percentage of people with low BMD was reliably highest in the group of patients aged 45–59 years (83.3%), while the number of people with fractures had no significant difference. In addition, in the older age groups, the percentage of patients with osteoproliferative changes increased. Thus, in the age category of patients of 45–59 and 30–44 years, syndesmophytes were detected 3.4 and 2.4 times more often (61.1% and 44.1%) compared to the group of patients aged 18–29 years (17.8%) (Table II).
Table II
State of bone mineral density in men with AS depending on age of patients
| BMD indicators | Age groups of patients | ||
|---|---|---|---|
| 18–29 years old (n = 28) | 30–44 years old (n = 59) | 45–59 years old (n = 18) | |
| Number of patients with low BMD [n (%)] | 6 (21.4%) | 23 (38.9%) | 15 (83.3%)*# |
| Number of patients with fractures [n (%)] | 4 (14.3%) | 6 (10.2%) | 2 (11.1%) |
| Number of patients with syndesmophytes [n (%)] | 5 (17.8%) | 26 (44.1%)* | 11 (61.1%)* |
| Lumbar spine | |||
| Z-score (M ±m) | –1.55 ±0.2 | –1.05 ±0.2 | –0.3 ±0.4 |
| T-score (M ±m) | –1.56 ±0.2 | –1.35 ±0.2 | –0.8 ±0.4 |
| BMD [g/cm2] | 0.91 ±0.02 | 0.95 ±0.02 | 1.00 ±0.01 |
| Femoral neck | |||
| Z-score (M ±m) | –0.91 ±0.1 | –0.94 ±0.1 | –0.87 ±0.2 |
| T-score (M ±m) | –1.9 ±0.3 | –1.4 ±0.2 | –1.5 ±0.2 |
| BMD [g/cm2] | 0.78 ±0.02 | 0.73 ±0.02 | 0.73 ±0.03 |
The research did not establish a significant association between the reduction of BMD and length of illness. In particular, the largest percentage of 59.1% (26) patients with low BMD was found in the group of patients with a duration of the disease from 5 to 10 years, while in this group the lowest average levels of Z- and T-score were observed both at the level of the lumbar spine and the level of the femoral neck. Similar data were obtained when analyzing the BMD index. With increasing time from the onset of the disease, higher bone tissue resynthesis processes was observed. Thus, in the group of patients with a disease duration of 5–10 years and more than 10 years, the percentage of people with syndesmophytes was significantly higher than in the group with a disease duration of up to 5 years (52.3–41.5% vs. 10%) (Table III).
Table III
State of bone mineral density in men with AS depending on duration of the disease
| BMD indicators | Groups according to duration of the disease | ||
|---|---|---|---|
| < 5 years (n = 20) | 5–10 years (n = 44) | > 10 years (n = 41) | |
| Number of patients with low BMD [n (%)] | 4 (20.0%) | 26 (59.1%) | 18 (43.9%)* |
| Number of patients with fractures [n (%)] | 3 (15.0%) | 5 (11.4%) | 4 (9.7%) |
| Number of patients with syndesmophytes [n (%)] | 2 (10.0%) | 23 (52.3%)* | 17 (41.5%)* |
| Lumbar spine | |||
| Z-score (M ±m) | –0.9 ±0.2 | –1.6 ±0.2* | –0.64 ±0.2# |
| T-score (M ±m) | –1.1 ±0.2 | –1.7 ±0.2* | –0.98 ±0.3# |
| BMD [g/cm2] | 0.96 ±0.02 | 0.91 ±0.01 | 0.98 ±0.03# |
| Femoral neck | |||
| Z-score (M ±m) | –0.6 ±0.1 | –1.1 ±0.1* | –0.89 ±0.1 |
| T-score (M ±m) | –0.7 ±0.3 | –1.5 ±0.1* | –1.5 ±0.12 |
| BMD [g/cm2] | 0.82 ±0.02 | 0.70 ±0.01* | 0.73 ±0.02 |
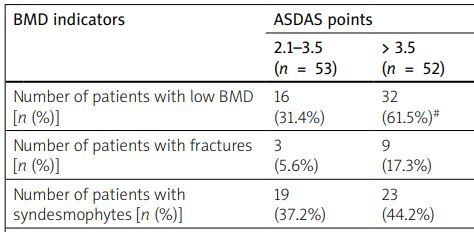
The research established that the decrease in BMD and the development of fractures were closely associated with high disease activity and low functional capacity, assessed by the ASDAS, BASDAI, and BASFI indices. Thus, in the group of patients with high activity (ASDAS 2.1–3.5), a decrease in BMD was found in 16 (31.4%) patients, in the group of patients with very high activity (ASDAS > 3.5) in 32 (61.5%) people, while in the group of patients with moderate AS activity (ASDAS 1.3–2.1), patients with a disorder of the structural and functional state of the bone were not detected at all. The average value of the Z-score at the level of the lumbar spine also probably decreased from the level of 1.1 ±.01 with moderate disease activity to the level of –0.7 ±0.2 in patients with high activity and –1.5 ±0.2 in patients with very high activity of the inflammatory process. At the level of the femoral neck, there was also a tendency to lose bone mass under conditions of high disease activity. We noted similar data for the BASDAI. In particular, in the group of patients with BASDAI less than 4 points, the percentage of people with low BMD and fractures was 21.2% and 3.1% of patients, and in the group with BASDAI above 4 points it was 43.1% and 15.3%, respectively. Despite the reliable dependence of low BMD on the high activity of the inflammatory process that we found, there was a proportion of patients in whom the structural and functional state of the bone tissue showed a rather weak association with the activity of the disease. Thus, with high activity according to the ASDAS and BASDAI indices, only a tendency for the percentage of patients with syndesmophytes to increase was observed. On the other hand, low functional capacity determined by the BASFI was closely related to osteoproliferative processes in bone tissue. Thus, an increase in the BASFI above 4 points caused a probable increase in the proportion of patients with syndesmophytes to 51.5% compared to 18.9% in patients with a BASFI of less than 4 points (Table IV).
Table IV
State of bone mineral density in men with AS depending on the indices of ASDAS, BASDAI and the functional index of BASFI
| BMD indicators | ASDAS points | BASDAI points | BASFI points | |||
|---|---|---|---|---|---|---|
| 2.1–3.5 (n = 53) | > 3.5 (n = 52) | < 4 (n = 33) | > 4 (n = 72) | < 4 (n = 33) | > 4 (n = 72) | |
| Number of patients with low BMD [n (%)] | 16 (31.4%) | 32 (61.5%)# | 7 (21.2%) | 31 (43.1%)* | 11 (29.7%) | 37 (54.4%)¥ |
| Number of patients with fractures [n (%)] | 3 (5.6%) | 9 (17.3%) | 1 (3.1%) | 11 (15.3%)* | 2 (6.1%) | 10 (13.9%) |
| Number of patients with syndesmophytes [n (%)] | 19 (37.2%) | 23 (44.2%) | 11 (33.3%) | 31 (43.1%) | 7 (18.9%) | 35 (51.5%)¥ |
| Lumbar spine | ||||||
| Z-score (M ±m) | –0.7 ±0.2 | –1.5 ±0.2# | –0.02 ±0.2 | –1.5 ±0.1* | –0.43 ±0.26 | –1.43 ±0.18¥ |
| T-score (M ±m) | –0.9 ±0.2 | –1.7 ±0.2# | –0.2 ±0.2 | –1.8 ±0.1* | –0.70 ±0.24 | –1.64 ±0.17¥ |
| BMD [g/cm2] | 0.98 ±0.02 | 0.89 ±0.02# | 1.05 ±0.3 | 0.89 ±0.02* | 1.01 ±0.03 | 0.90 ±0.02¥ |
| Femoral neck | ||||||
| Z-score (M ±m) | –0.8 ±0.1 | –1.01 ±0.1 | –0.5 ±0.1 | –1.1 ±0.1* | –0.61 ±0.12 | –1.07 ±0.09¥ |
| T-score (M ±m) | –1.2 ±0.1 | –1.7 ±0.2 | –1.01 ±0.1 | –1.5 ±0.1* | –1.03 ±0.12 | –1.54 ±0.09¥ |
| BMD [g/cm2] | 0.75 ±0.02 | 0.73 ±0.01 | 0.79 ±0.02 | 0.72 ±0.01* | 0.79 ±0.02 | 0.72 ±0.01¥ |
A decrease in BMD and, accordingly, an increase in the number of people with fractures, osteoporosis, and osteopenia, was also quite closely associated with the content of CRP in blood serum. Thus, in patients with an optimal level of CRP, the average value of the Z-score at the level of the lumbar spine was 0.30 ±0.19, and in people with extremely high and high levels of CRP the values were –1.24 ±0.2 and –1.77 ±0.4, respectively, which is 6 times lower. Similar data were found at the level of the femoral neck, where the average values of Z- and T-scores were the lowest in conditions of high CRP activity. As for the BMD indicator, at the lower back level, it was 17.3% (thighs – 18.8%) less compared to that at the optimal level of CRP and was equal to 0.87 ±0.04 g/cm2 (0.71 ±0.02 g/cm2) vs. 1.07 ±0.01 g/cm2 (0.80 ±0.01 g/cm2). Among patients with an optimal level of CRP, a decrease in BMD and the development of fractures were found in 26.1% and 4.3% of people, with an extremely high level of CRP – 39.3% and 7.1%, respectively. In men with high activity of the inflammatory process (CRP > 13.4), the proportion of patients with low BMD and fractures was 76.9% and 26.9%. Osteoproliferative processes in bone tissue did not significantly depend on the level of CRP (Table V).
Table V
The state of bone mineral density in men with AS depending on the content of C-reactive protein
| BMD indicators | Content of CRP | ||
|---|---|---|---|
| < 5.4 ng/l (n = 23) | 5.4–13.4 ng/l (n = 56) | > 13.4 ng/l (n = 26) | |
| Number of patients with low BMD [n (%)] | 6 (26.1%) | 22 (39.3%) | 20 (76.9%)*# |
| Number of patients with fractures [n (%)] | 1 (4.3%) | 4 (7.1%) | 7 (26.9%)*# |
| Number of patients with syndesmophytes [n (%)] | 10 (43.5%) | 25 (44.6%) | 7 (26.9%) |
| Lumbar spine | |||
| Z-score (M ±m) | 0.30 ±0.19 | –1.24 ±0.18* | –1.77 ±0.39* |
| T-score (M ±m) | –0.21 ±0.07 | –1.47 ±0.16* | –1.93 ±0.34* |
| BMD [g/cm2] | 1.07 ±0.01 | 0.93 ±0.02* | 0.87 ±0.04* |
| Femoral neck | |||
| Z-score (M ±m) | –0.40 ±0.03 | –1.01 ±0.09* | –1.14 ±0.17* |
| T-score (M ±m) | –0.98 ±0.03 | –1.41 ±0.10* | –1.60 ±0.17* |
| BMD [g/cm2] | 0.80 ±0.01 | 0.74 ±0.01* | 0.71 ±0.02* |
Additional confirmation that the violation of the structural and functional state of bone tissue depends on the course of the disease and the activity of the inflammatory process was obtained from the paired correlation analysis. It was established that there were statistically significant negative relationships between clinical and laboratory markers of inflammatory process activity on the one hand and low BMD on the other. Thus, the density of bone tissue at the level of the lumbar spine and femoral neck was closely associated with the total indicators of disease activity and severity according to ASDAS (from –0.20 to –0.46, p < 0.05), BASDAI (from –0.29 to –0.65, p < 0.05) and BASFI (from –0.26 to –0.59, p < 0.05). There were also probable associations between the level of CRP and densitometric indicators (BMD and Z-score) of both studied areas (from –0.28 to –0.35, p < 0.05) (Table VI).
Table VI
Correlation coefficients of BMD indexes with risk factors and disease course in men suffering from AS
| Indicator | Bone mineral density | |||||
|---|---|---|---|---|---|---|
| BMD [g/cm2] | Z-score | T-score | ||||
| Lumbar spine | Femoral neck | Lumbar spine | Femoral neck | Lumbar spine | Femoral neck | |
| Age | 0.14 | –0.19 | 0.16 | –0.04 | –0.23* | –0.13 |
| Disease duration | 0.18 | –0.11 | 0.20* | 0.05 | –0.19 | –0.44* |
| ASDAS | –0.39* | –0.27* | –0.32* | –0.20* | –0.46* | –0.21* |
| BASDAI | –0.51* | –0.32* | –0.49* | –0.29* | –0.65* | –0.45* |
| BASFI | –0.32* | –0.35* | –0.26* | –0.27* | –0.59* | –0.59* |
| CRP | –0.35* | –0.36* | –038* | –0.28* | –0.14 | 0.03 |
Discussion
Conducted for the first time in the Podilsk population of AS patients, the study of the structural and functional state of bone tissue showed that a decrease in BMD at the level of the lumbar spine and femoral neck was detected in 45.7% of patients. In particular, in men with AS under the age of 50, low bone density, determined by the Z-score, was found in every third patient, while at the level of the lumbar spine it was found 4.8 times more often (33.3%) than at the level of the femoral neck (6.9%). In patients with AS over 50 years of age, BMD analysis (according to the T-score) showed that 44.4% of patients were diagnosed with osteopenic syndrome at the level of the lumbar spine, and 72.2% of patients at the level of the femoral neck. The percentage of patients with osteoporosis at the level of the femoral neck and lower back was 11.1–16.7%. Regarding the analysis of changes in BMD by AS, there are numerous publications in the literature. The prevalence of osteoporosis associated with AS has been reported to range from 19% to 62%, and that of osteopenia from 50% to 92%. Thus, in the study by Wang et al. [13], osteoporosis and osteopenia were detected in 21% and 62.8% of AS patients. According to Vasdev 8.7% and 11.5% of patients were diagnosed with osteoporosis at the lumbar and femoral neck levels [14]. In the study of Van Der Weijden [7], osteopenia at the level of the lumbar spine was detected in 54% of patients, at the level of the femoral neck in 51% of people, and osteoporosis in the same studied areas in 13 and 16% of cases, respectively. Similar patterns were observed in the study of Malochet-Guinamand [12], where a decrease in BMD was found in 55% of patients, among them osteoporosis – in 6.7% of cases. In our opinion, such variability of results probably depends on taking into account the presence or absence of osteoproliferative changes in patients with AS and the wide range of methods used to assess BMD. After all, in the above-mentioned studies, the percentage of patients with syndesmophytes varies from 7 to 55% [7, 12, 14]. In some studies [15] the presence of syndesmophytosis was an exclusion criterion, while others [16, 17] did not take them into account. According to our data, pathologically new formation of bone tissue occurred in 40% of patients.
Changes in BMD had no clear relationship with the age of the patients. Thus, the average values of Z-score, T-score and BMD at the level of the lumbar spine was significantly higher in the older age group, while at the level of the femoral neck there were no associations with age. In addition, in men of the older age group, compared to the youngest patients, there were more people with syndesmophytes (61.1 vs. 17.8%).
Similar patterns were found in other studies. In particular, according to Klingberg et al. under the age of 50, BMD was below the expected age norm in 5% of patients, while in the age group older than 50, osteoporosis and osteopenia were diagnosed in 27% and 34% of individuals, respectively [4]. According to Hu [18], the age of patients is one of the most significant risk factors for the development of osteoporosis. However, there are many studies in which the age of patients is not associated with a decrease in BMD; instead, it was closely connected with syndesmophytes [19, 20].
We found that changes in bone tissue were associated with duration of the disease. In particular, with increasing time from the onset of the disease, the number of people with syndesmophytes increased. Thus, in the group with a disease duration of more than 10 years, syndesmophytes were found in 41.5% of people, while in the group with a disease history of up to 5 years, there were only 10% of them. On the other hand, the decrease in BMD was not associated with the duration of the disease, since the lowest proportion of patients with osteodestructive changes (59.1%) was found in the group of patients with a disease duration of 5 to 10 years, and in the group with a disease duration of more than 10 years, there were fewer such patients (43.9%). Therefore, it can be assumed that an increase in the duration of the disease contributes to the development of osteoproliferative changes in AS. The obtained data are consistent with the results of previous research [21].
Inflammation is the main mechanism of bone loss in AS. According to our data, the process of BMD reduction in men with AS is closely associated with high activity of the inflammatory process determined by the ASDAS and BASDAI indices, and the pro-inflammatory inflammatory mediator CRP. Thus, in persons with high values of the ASDAS and BASDAI indices, the average values of Z-score, T-score, and BMD level at the lower back level were significantly lower, and the proportion of patients with low BMD was two times higher than in patients with low activity of the inflammatory process. Osteoproliferative changes had a rather weak association with disease activity. We found only a trend towards an increase in the proportion of patients with syndesmophytes with high activity according to the ASDAS and BASDAI indices, and no correlation was found with regard to the level of CRP. The role of systemic inflammation as a predictor of generalized bone loss is also confirmed by most scientists [22, 23]. An increase in markers of the inflammatory process (ASDAS, BASDAI, CRP, ESR) has also been reported in patients with osteoproliferative changes [24, 25]. However, in one study [26] it was found that the development of osteoproliferative and osteodestructive changes occurred regardless of the presence of an inflammatory process.
Along with the high activity of AS, in the development of changes in bone mass, a decrease in functional status due to limited mobility of the spine assumes great importance [27]. In our study, low functional capacity determined using the BASFI had a negative effect on bone density. In particular, in the group with preserved functional status (BASFI < 4), 29.7% of people had destructive changes in bone tissue, while in the group with low functional status capacity (BASFI > 4) there were 54.4% of them. In addition, in the latter group, a probable increase in the proportion of patients with syndesmophytes was observed to 51.5%, compared to 18.9% in patients with a BASFI of less than 4 points.
Disorder of the structural and functional state of bone tissue due to AS significantly increases the risk of vertebral compression fractures [4, 12]. We found that low-energy fractures had occurred in 11.4% of patients. The study did not find a relationship between age, duration of the disease, and the presence of low-energy fractures. Instead, the activity of the inflammatory process (according to ASDAS, BASDAI, and CRP) tended to increase in individuals with fractures. Similar data were obtained in a study by Kim et al. [11]; in particular, a high inflammatory process (according to CRP) and GC intake were associated with a high risk of osteoporotic fractures, while age and duration of the disease were not significant factors. According to data in the Kang et al. study [28], high levels of pro-inflammatory markers are predictors of the development of fractures in AS patients.
Previous studies have shown that a decrease in BMD is a predictor of the development of fractures in AS patients [12, 29]. However, it is quite difficult to clearly determine BMD indicators of the lumbar spine using DEXA in the anteroposterior projection, precisely because of the presence of syndesmophytes [4]. The trabecular bone score (TBS) is a recently developed tool to assess bone strength. Previous studies have shown that TBS can predict fractures regardless of BMD values and has additional value in assessing the structural and functional state of bone tissue [8, 30].
Conclusions
Bone tissue changes in the form of syndesmophytosis were detected in 40% of patients with AS and were associated with the duration of the disease, age of the patients and low functional capacity. The presence of syndesmophytes negatively affected BMD indicators; in particular, the average values of Z-score, T-score and BMD index at the level of the lumbar spine were normal or even high in individuals with osteoporosis/osteopenia at the level of the femoral neck.
A decrease in BMD (according to the Z-score or T-score) was found in 41.9% of men with AS, and only in 17.2% of the control group and mostly associated with high activity of the inflammatory process according to ASDAS, BASDAI (r = –0.39; –0.65), CRP (r = –0.28, –0.38) and low functional capacity according to BASFI (r = –0.27, –0.59), while age and disease duration were risk factors for the progression of osteodestructive changes only at the level of the femoral neck.
Low-energy fractures occurred in 11.4% of men with AS. The presence of fractures was associated with high disease activity (ASDAS, BASDAI, CRP), because fractures mostly occurred in individuals with the highest values of the activity indices ASDAS, BASDAI and the pro-inflammatory marker CRP, and had no relationship with syndesmophytosis, age or duration of the disease.