Introduction
Autoimmune diseases (ADs) are pathologic conditions resulting from an immune system malfunction and affecting 3–10% of the general population [1]. They are characterized by the production of high-affinity autoantibodies which target the “self” molecules of any part of the body [2, 3]. Among others, the hematopoietic system can be affected, leading to peripheral cytopenias. Myelodysplastic syndrome (MDS) is a disorder affecting the bone marrow stem cells [4, 5]. It is characterized by dysmyelopoiesis, affecting erythroid, myeloid and megakaryocytic lineages to variable degrees [6]. Therefore, anemia, neutropenia and/or thrombocytopenia are its main clinical manifestations [7].
The association between ADs and MDS has been widely recognized [8]. However, little is known about the exact mechanism of this association and the causal link has yet to be determined. In addition, the non-rare frequency of cytopenias seen with ADs makes the diagnosis of an associated MDS challenging in some situations.
The aim of this review is to study the clinical association between MDS and ADs and analyze its pathogenic, therapeutic, and prognostic aspects, so as not to miss the diagnosis of an underlying MDS when dealing with ADs.
Epidemiological data and diagnostic delay
Autoimmune diseases affect 5–10% of the world’s population and can sometimes result in morbidity and mortality rates as high as those seen in cardiovascular diseases or cancers [9]. To date, there have been identified over 80 types of ADs, half of which are considered rare [9]. A clear female predominance is noted [1] and the median age of onset depends on the disease.
In contrast, MDS is considered to be a disease of the elderly, most often males with a median age of diagnosis around 70 years [4].
Autoimmune diseases and MDS can be associated. They may be diagnosed either simultaneously or with a time leg. Several studies evaluating the risk of myeloid hematologic malignancies in ADs have proved a significant excess of MDS cases among several ADs, such as systemic lupus erythematosus, rheumatoid arthritis and inflammatory bowel diseases, of which Crohn’s disease was the most frequent [10]. This risk is reported to be 2.1 times higher in patients with a history of AD according to a Swedish study [11, 12]. Likewise, ADs have been found to be more frequent in patients with MDS when compared with controls [8], with a frequency of about 10–30% [13–15].
In the case of concomitant MDS and AD, a younger median age of diagnosis of MDS and a less marked male predominance compared to patients with isolated MDS have been reported [16].
Regarding diagnosis time, the chronology of onset of ADs and MDS is variable. Indeed, immunological disorders may rarely precede the onset of MDS by several months to years [8, 17] even more if the AD has lasted 10 years or longer [18]. In other cases, these two entities may coexist and be diagnosed simultaneously. However, in the majority of cases, MDS characterizes the initial clinical presentation. A median time of 8 months between the diagnosis of MDS and AD was reported [15].
Clinical features
Concerning clinical characteristics, ADs are more common in female MDS patients [19]. They have variable and often incomplete features, which sometimes makes them difficult to classify. Moreover, MDS-associated ADs are characterized by less favorable baseline characteristics compared with MDS without autoimmune manifestations [15].
According to a recent case-control study of 788 patients conducted by Mekinian et al. [15], the WHO (World Health Organization) MDS subtypes associated with ADs are mainly MDS with excess blasts (DS-EB) and MDS with single lineage dysplasia (MDS-SLD). This association was higher in cases of very low-risk levels based on the Revised International Prognostic System (R-IPSS) [19].
Median levels of blood count parameters, particularly hemoglobin, platelets, and neutrophils, appear to be similar in patients with and without ADs. However, a higher number of blasts was found in patients with an associated AD [15]. It is also interesting that autoantibodies, without clinical signs of AD, have been described in more than 50% of patients diagnosed with MDS (20% of anti-nuclear antibodies without any specificity, 9% of anti-neutrophil cytoplasmic antibodies (ANCA) without any specificity, 12% of rheumatoid factor (RF) and 13% of anti-tissue antibodies) [11]. More interestingly, similar frequencies of autoantibodies have been found in (age-paired) patients without MDS, except for anti-parietal cells. This means that, on the practical side, autoantibodies screening would not be of much help for the diagnosis of MDS-related autoimmune manifestations [11]. Added to that, peripheral cytopenias are not rarely seen with ADs, which may delay the diagnosis of an associated MDS. The appearance of cytopenias outside the attacks of ADs, their persistence under treatment and despite the good evolution of the AD symptoms as well as their central character must alert clinicians to a possible coexistent hematologic disease, especially an MDS. Thus, in case of any doubt, a bone marrow aspirate must be performed. On the other hand, if the patient is already diagnosed with MDS, an additional AD is not always easy to diagnose. This is mainly due to the great clinical heterogeneity of ADs as well as overlapping conditions such as bone marrow infiltration, chemotherapy and/or transfusion support [20]. Notably, special attention should be to immune-mediated hemostatic disorders that may be associated with MDS, such as autoimmune hemolytic anemia (AHAI), immune thrombocytopenic purpura (ITP) and catastrophic antiphospholipid syndrome (CAPS) [20].
The most frequently described autoimmune diseases in association with myelodysplastic syndrome
Various autoimmune manifestations, being part of defined clinical entities or representing an unclassified or isolated organ involvement, can be observed in association with MDS.
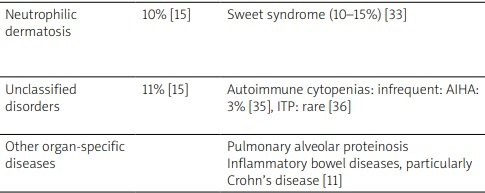
Interestingly, Saif et al. [21] classified these autoimmune manifestations into five entities: systemic vasculitis, connective tissue disorders, isolated autoimmune phenomena, immune-mediated hematological abnormalities and asymptomatic serological immunologic abnormalities. Table I summarizes the frequencies of association of these autoimmune manifestations with MDS as well as their particularities. As shown, vasculitis and connective tissue disorders, followed by inflammatory arthritis, are the most frequently described autoimmune conditions in patients with MDS [22–36]. As for limited clinical manifestations, the most commonly reported one is hypothyroidism followed by rheumatic polymyalgia, with frequencies of 25.6% and 15.4% of patients, respectively [19].
Table I
Autoimmune diseases associated with myelodysplastic syndrome
| Subsets of systemic MDS-related ADs | Frequency | Manifestations | Particularities |
|---|---|---|---|
| Vasculitis | 32% [11] | HBV-negative polyarteritis nodosa (30%) [11] Giant cell arteritis (23%) [11] ANCA-negative leukocytoclastic vasculitis (10–30%) [22, 23] Behçet’s like syndrome, usually with incomplete forms and predominant digestive involvement (15–20%) [24–26] ANCA-associated vasculitis, Takayasu’s arteritis and IgA vasculitis: exceptionally reported [11] | Association with more impaired kidney function, more GC dependency and less complete remission than solid tumor-associated vasculitis [27] |
| Connective tissue disorders | 25–30% [15] | Relapsing polychondritis (60%) [15] Systemic lupus erythematosus (30%) [15] Sjögren’s syndrome (SS) or myositis: exceptionally reported [16, 23] Systemic sclerosis: never reported [16, 23] | Skin lesions in relapsing polychondritis are more frequent, particularly neutrophilic dermatosis [28, 29] |
| Inflammatory arthritis | 23% | Undifferentiated arthritis: the most frequent entity and typically present as polyarticular and symmetrical arthritis, usually without structural progression [15] Polymyalgia rheumatica (10%) (sometimes associated with giant cell arteritis [16, 30, 31] | Patients tend to be older with male dominance and more involved joints [32] Undifferentiated arthritis typically present as symmetrical polyarticular arthritis, usually without structural progression [15] |
| Neutrophilic dermatosis | 10% [15] | Sweet syndrome (10–15%) [33] | Sweet syndrome can be induced by G-CSF use or chemotherapy [34]. It is associated with a worse prognosis of MDS [33] |
| Unclassified disorders | 11% [15] | Autoimmune cytopenias: infrequent: AIHA: 3% [35], ITP: rare [36] | AIHA is more often described with low-risk MDS, as MDS-SLD or MDS-RS [24] |
| Other organ-specific diseases | Pulmonary alveolar proteinosis Inflammatory bowel diseases, particularly Crohn’s disease [11] |
[i] ADs – autoimmune diseases, AIHA – autoimmune hemolytic anemia, ANCA – antineutrophil cytoplasmic antibodies, G-CSF – granulocyte colony-stimulating factor, GC –glucocorticosteroid, HBV – hepatitis B virus, IgA – immunoglobulin A, ITP – immune thrombocytopenic purpura, MDS – myelodysplastic syndrome.
Causality link/physiopathology
The fortuitous or coincidental relationship of this association could be raised but seems to be less plausible given the non-negligible frequency of coexistance of these two. However, not much is known about whether one disease is partly responsible for the development of the other. Indeed, despite the identification of several possible factors contributing to this association, no hypothesis in particular was supported. It might be more reasonable to study the pathophysiological basis of this association by considering the chronology of installation of the two entities. In cases where AD characterizes the initial clinical presentation, susceptibility to MDS is the result of impaired immune surveillance [10]. Indeed, a chronic immunologic dysregulation with a permanent immune stimulation resulting in bone marrow lesions or infiltration is suggested [8, 10]. However, in this context, drug etiology is the most adopted and MDS is thought to be a therapy-related myeloid neoplasm arising as a delayed effect of treatment of AD [10]. In effect, immunosuppressive therapy used in ADs includes several drug classes, notably antimetabolites (such as azathioprine, methotrexate and 6-mercaptopurine) and alkylator agents (such as cyclophosphamide and DNA-topoisomerase II inhibitors/mitoxantrone) [10]. These treatments are responsible for many molecular mechanisms that underpin leukemogenesis but without a proved correlation between the risk of myeloid neoplasms and the duration of drug exposure [37]. Of note, the carcinogenic potential was best documented with azathioprine, followed by cyclophosphamide and to a lesser extent with mitoxantrone [37–39].
In cases where MDS precedes immunological manifestations, ADs are thought to result from MDS-related immune dysregulation disorders [40]. Thus, a deficient tolerance including both cellular and humoral immunity [7] may be associated with reduced anti-tumor responses and could lead to the emergence of autoreactive cells and hence to impaired immune regulation [11]. Increased cytokine production, mainly of interleukin-1 and -6 (IL-1 and IL-6) and tumor necrosis factor α (TNF-α), by malignant monocytes principally in chronic myelomonocytic leukemia or from the lymphocytes being part of the abnormal dysplastic clone, has also been reported [41]. However, further studies are needed to better understand the exact mechanisms that induce ADs in MDS patients.
In the context of concurrent AD and MDS, shared trigger factors and/or genetic susceptibilities between the two disorders is then evoked [10, 19]. Hochman et al. even raised the question whether MDS and ADs and systemic inflammatory disorders are actually “two sides of the same coin” based on the idea of a likely shared underlying disease state that may manifest as an autoinflammatory disorder, a myeloid neoplasm, or both [40]. As for environmental factors, exposure to some chemical products such as tobacco has been identified as a risk factor of occurrence of both MDS and ADs [42, 43].
Therapeutic options
Treatment of MDS-associated ADs is not codified. The main challenge here is to achieve remission of the autoimmune manifestations without aggravating MDS-related cytopenias. Glucocorticosteroids (GCs) alone, as a first-line treatment, have been reported to be effective against autoimmune manifestations in up to 80% of cases in some series [15]. However, this response is not always complete or durable, and high rates of steroid-dependent or refractory cases have been reported [44]. Moreover, MDS’ responses to GCs used to treat autoimmune conditions are variable. Cases of improved, refractory or even worsened cytopenias have been registered [15–17].
Immunosuppressive treatments can be used as second-line therapies. However, in most cases, no hematological response has been reported but rather a worsening of the MDS-related cytopenias with all the ensuing complications, especially infectious ones [15, 16, 45].
Biologic targeted treatments are also a possible therapeutic option for MDS-associated ADs but with lower response rates compared to GCs and compared to non MDS-related ADs [25]. Moreover, they have no effect on cytopenias of the underlying MDS [25, 46]. Rituximab proved to be the most efficient among biologics, with an overall response of 58%, mainly in vasculitis. However, a high rate of adverse effects was reported with these therapies [25]. In contrast, MDS treatment, in particular azacitidine, has been found to have a good effect on associated ADs with regard to the dose of GCs and the number of steroid-dependent patients [15]. As for hypomethylating agents, their efficacy in terms of outcome in MDS-associated ADs has to be confirmed. Interestingly, hematopoietic stem cell transplantation (HSCT), which is the only satisfactory option reported for MDS management, is currently also considered as an effective option in treating severe, refractory AD through reconstituting the hematopoietic system and restoring immune functions as well [47, 48].
Prognostic specific aspects
The heterogeneity of ADs associated with MDS makes the prognostic significance of this association controversial. However, in general, whereas the AD develops before the MDS or during its course, no differences in overall survival or in the rates of acute myeloid leukemia and death have been reported in patients with ADs and MDS compared to with patients with MDS [8, 11, 20]. Nonetheless, systemic vasculitis has been reported as a poor prognostic factor in patients with MDS, mainly due to the presence of high-risk MDS features, and Sweet syndrome has been associated with progression of MDS [11]. Cryoglobulinemic vasculitis was also associated with lower median overall survival [17] due to a higher infectious rate [20]. However, in the presence of an associated AD, the underlying cytogenetic features of MDS do not seem to be influenced [11].
Conclusions
Myelodysplastic syndrome and ADs may coexist or complicate each other with a delay of months to years. Myelodysplastic syndrome-associated ADs have some epidemiological and clinical specific aspects but no major effects on survival. Although this association has been established by several studies, it is sometimes difficult to diagnose, and MDS should always be considered in the diagnostic algorithm of patients having ADs when cytopenia(s) cannot be explained by the immunological disorders.