Introduction
Psoriatic arthritis (PsA) is an inflammatory disease characterized with skin and joints involvement. It is estimated to occur in nearly 1/3 of patients with psoriasis [1]. Psoriatic arthritis is part of the seronegative subgroup of spondyloarthritis and may be present equally in both men and women [2].
Psoriatic arthritis is associated with axial and/or peripheral joints involvement and may manifested with various clinical symptoms such as recurrent episodes of enthesitis/dactylitis, mono-/oligoarthritis, destructive polyarthritis, and spondylitis [3].
Other manifestations, such as cardiovascular disease, metabolic syndrome and eye involvement may also seen [4]. Salusins are endogenous bioactive peptides with hemodynamic and mitogenic activities [5].
In humans, salusins consist of two related peptides as salusin-α and salusin-β with 28 and 20 amino acids, respectively and are produced from the same precursor, preprosalusin [6].
Although the underlying mechanisms are not known in detail, it has been reported that both salusins (salusin-α and salusin-β) may be associated with the development and progression of atherosclerosis [7]. The studies showed that while salusin-β has pro-atherosclerotic effects salusin-α has anti-atherosclerotic effects [8].
Salusins are present in human plasma and urine and expressed from vessels, kidneys and central nervous system, as well as from monocytes and macrophages [9, 10]. Foam cells which are formed when macrophages phagocytize oxidized LDL in the intima, are stimulated by salusin-β but suppressed by salusin-α [11]. It has also been suggested that salusins may play an important role in regulation of the immune system and inflammation [12].
Studies in the literature investigating the possible role of salusins in the pathogenesis of rheumatic diseases are still not sufficient yet. The increased serum salusin-α levels and correlation with various disease parameters have been reported in patients with rheumatoid arthritis (RA) and Behçet’s disease (BD) [13, 14].
The aim of our study was to analyze the serum salusin-α and salusin-β levels in PsA patients and to determine their possible relationship with the disease findings.
Material and method
Forty patients with PsA who fulfilled the CASPAR criteria and forty age- and gender-matched healthy volunteers were included in the study. Demographic and clinical data were recorded in all patients.
Disease activity indexes (PASI, BASDAI, BASFI, HAQ) were recorded and routine laboratory tests and genetic testing for HLA-B27 were performed. Serum samples were collected after their consent had been taken and stored at –20°C prior to analysis.
The serum salusin-α and salusin-β levels were examined using human salusin-α kit (Cat No. CEB892Hu, Cloud-Clone Corp., Houston, USA) and human salusin-β kit (Cat No. CEC026Hu, Cloud-Clone Corp., Houston, USA) by enzyme linked immunesorbent assay (ELISA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using the RStudio software version 0.98.501 via R language and the graphs were prepared via Apache Open Office 4.1.1.
The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk test) to determine whether they were normally distributed.
Descriptive analyses were presented using the mean, standard deviation and standard error of the mean (S.E.M.) for normally distributed variables. Independent t-test was used to compare the measurements in control and patient groups for measurement of values. A p-value of less than 0.05 (p < 0.05) was considered to show a statistically significant result.
Results
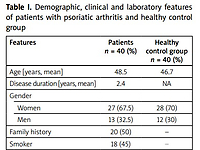
Forty patients who were admitted to the rheumatology outpatient clinic and 40 healthy volunteers were included in the study. The age range was 25–52 years in PsA patients and 23–50 years in the control group. The mean age of PsA patients was 48.5 years and 47 years in the control group. Among 40 PsA patients, 13 (32.5%) were male and 27 (67.5%) were female and the mean duration of disease was 2.4 years.
The female/male ratio in the control group was 28/12. There was no significant difference in age, gender or demographic distribution between the groups (p > 0.05).
Demographic and clinical assessment of PsA patients were as follows: 18 (45%) were smokers, 19 (47.5%) had HLA-B27 positivity, 33 (82.5%) had sacroiliitis, 36 (90%) had enthesitis, 23 (57.5%) had DIP joints and nail involvement, 26 (65%) had wrist involvement, and 11 (27.5%) had ankle involvement.
Family history was positive in 20 (50%) PsA patients, while none of the healthy controls had history of psoriasis/PsA in their families. Twenty (50%) and 25 (62.5%) patients were found to have elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels, respectively (Table I).
Table I
Demographic, clinical and laboratory features of patients with psoriatic arthritis and healthy control group
Twenty-four (60%) PsA patients received methotrexate (MTX) treatment, 6 (15%) PsA patients received low-dose corticosteroids, and 8 (20%) patients received biologic agents (7 anti-TNF-alpha inhibitors, 1 anti-IL-17 inhibitor). Two patients (5%) did not receive any treatment.
In this study, it was found that the PsA patients had a higher mean serum salusin-α level (p = 0.004) compared with the control group of cohorts (Table II).
Table II
Serum salusin-α and salusin-β levels in patients with psoriatic arthritis and control group
| Variables | Control group (n = 40) | PsA patients (n = 40) | P-value |
|---|---|---|---|
| Salusin-α | 105 pg/ml | 431.5 pg/ml | 0.004 |
| Salusin-β | 93.9 pg/ml | 79.2 pg/ml | 0.285 |
Although mean serum level of salusin-β was found to be slightly lower in PsA patients than the healthy controls, the difference was not statistically significant (p = 0.285). In analysis of gender distribution, the mean serum salusin-a level was higher in women than in men, but this difference was not significant.
A relationship was found between mean serum salusin-α level and ankle arthritis (p = 0.04). Regarding laboratory parameters a relationship was found between serum CRP and serum salusin-α level, while no correlation with ESR was observed (p = 0.03 and p = 0.326 respectively).
However, no correlation was observed between mean serum salusin-α level and other disease activity and functional indices (BASDAI, BASFI, HAQ) examined in this study. There was no correlation between serum salusins levels and HLA-B27 positivity.
No significant difference was found between salusin-α and salusin-β and median PASI scores (p = 0.154, p = 0.132 respectively). Similarly, apart from a positive relationship with ankle arthritis (p = 0.02) no statistically significant correlation was observed between mean serum salusin-β level and other disease activity indices.
Discussion
In this study, we found that the PsA patients had higher mean salusin-α level compared to the healthy control group. However, mean serum salusin-β level was similar between the PsA patients and healthy subjects.
To our knowledge, there has been no study in the literature investigating the levels of both salusin-α and salusin-β in serum samples from PsA patients. There are few studies in literature investigating the relationships between rheumatic diseases and serum salusins levels.
Ozgen et al. [13] investigated the level of serum salusin-α in RA and Behçet’s disease (BD) patients and found that mean serum level of salusin-α was higher in both patient groups in comparison with the healthy control group, which is in line with the findings of our study. Detection of an elevated serum salusin-α level in PsA patients is very significant due to the anti-atherogenic effects of salusin-α.
Erden et al. [14] found that mean serum level of salusin-α was lower in BD patients and it was reported to be even much lower in BD patient with metabolic syndrome. Since our present study did not involve BD patients we have no data to discuss this discrepancy further.
However, the researchers also reported that the mean serum salusin-β level was higher in BD patients. This finding may appear to contradict our study in which a similar level of salusin-β was found in PsA patients and healthy controls.
The reasons for this may be due to several factors e.g. either the level of salusin-β in sera is normally not affected in PsA patients or the sensitivity of the salusin-β to proteases as a peptide hormone.
Koca et al. [15] reported that salusin-α levels were lower in systemic lupus erythematosus and systemic sclerosis patients than in healthy subjects. The authors found no correlation of salusin-α with disease activity indices and they hypothesized that the decreased salusin-α levels may contribute to subclinical atherosclerosis.
It has been demonstrated that salusin-α and salusin-β are expressed by inflammatory cells which may partly explain why a high level of serum salusin-α was detected in the present study as PsA is a chronic inflammatory arthritic disease [16].
It is considered that serum salusin-α levels are not generally affected by various physiological conditions but strongly affected by the severity of atherosclerotic cardiovascular diseases.
Serum salusin-α levels are reported to be notably lower in patients with coronary artery disease (CAD) compared to non-CAD patients, indicating the possible role of serum salusin-α as a reliable biomarker for detection of CAD [17].
Watanabe et al. [18] also studied the correlations between serum salusin-α levels and carotid atherosclerosis in hypertensive patients and found significantly lower level of salusin-α and higher values of intima-media thickness (IMT) and plaque score, suggesting a negative correlation between serum salusin-α levels and maximal IMT.
Investigation of the reasons for decreased production of salusin-α in atherosclerotic lesions revealed that salusin-α expression may be inhibited by the activation of JAK-2 kinase [19].
This enzyme is believed to be upregulated by angiotensin II, inflammatory cytokines, and atherogenic growth factors in atherosclerotic and hypertensive diseases [20].
The elevation of serum salusin-α levels in PsA patients as observed in the present study can be very important in terms of indicating the possible role of serum salusin-α as a reliable biomarker for PsA patients [21].
In addition, increased serum salusin-α levels in PsA patients can imply prevention and/or slow progression of atherosclerosis in these patient groups given the proven effect of the salusin-α as an anti-atherogenic peptide [22].
Conclusions
In conclusion, in the present study we found elevated levels of serum salusin-α in PsA patients, compared to control healthy subjects. This observation may be attributable to several reasons but production of salusin-α by inflammatory cells may partly explain it.
Notably, elevated serum salusin-α levels may indicate the possible potential role of this peptide as a reliable biomarker in PsA patients.
However, further research involving more study subjects and multicenter prospective studies are needed to explain and clarify this observation.