Introduction
Sjögren’s syndrome (SS) is a multisystem autoimmune disorder characterized by inflammation of the exocrine glands such as the salivary and lacrimal glands. Usually symptoms include dryness of the eyes, mouth, and skin [1, 2]. This syndrome occurs predominantly in women (female-to-male ratio 9 : 1) [3–5], with a prevalence rate of 0.1–4.8% [3, 6, 7].
Sjögren’s syndrome is one of the most common autoimmune diseases, and it may begin at any age but affects women predominantly in the fourth decade of life [6, 8]. A higher frequency of SS has been observed in pregnant women in recent years, probably due to the advanced maternal age of the first gestation [5], and better knowledge of the disease with early diagnosis [5].
This syndrome may present as a primary condition (primary SS), but often accompanies other autoimmune disorders (secondary SS), most commonly rheumatoid arthritis (AR) or systemic lupus erythematosus (SLE) [5–7].
Data regarding the correlation between SS and pregnancy outcomes are still limited and contradictory. However, like other autoimmune diseases, SS seems to be associated with a higher risk for adverse maternal and fetal outcomes [8, 9].
The most severe outcome is congenital heart block (CHB) [6, 7]. It is related to transplacental passage of maternal anti-Ro/SSA and anti-La/SSB antibodies, which may mediate tissue damage of the atrioventricular (AV) node [7]. It occurs in approximately 1–2% of offspring of a mother with anti-Ro/SSA antibodies [4, 5, 7]. Other adverse outcomes associated with SS are an increased risk of miscarriage and fetal loss [6, 7].
The primary aim of the present study was to evaluate the pregnancy and fetal outcomes in patients with SS. The secondary aim was to perform a case-control study including a group of healthy women with singleton pregnancies who attended the same hospital and during the same period to investigate the impact of SS pregnancy and neonatal outcomes.
Material and methods
Study setting and participants
This descriptive and retrospective case-control study included a total of 26 pregnancies in women with a diagnosis of SS, with antenatal follow-up, delivery, and puerperium at the Obstetric Department of our institution, a tertiary Portuguese university hospital (Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto), between January 2015 and December 2020.
Each pregnancy was considered an event. Multiple gestations or elective terminations for personal reasons were excluded. This retrospective analysis included a control group (CG) of 109 pregnant women with antenatal care and delivery in our institution. The CG was randomly selected from healthy women with singleton pregnancies who attended the same hospital and during the same period.
Variables, fetal and obstetric outcomes
In both groups, baseline maternal data were collected: age, past obstetric history, and medical antecedents. Maternal and fetal outcomes were assessed: gestational age at delivery, cesarean delivery, birth weight, and Apgar score at 1 and 5 min after birth, admission to the neonatal intensive care unit (NICU), development of neonatal congenital heart block (CHB), presence of congenital malformation and obstetric complications (preterm delivery [PD], preeclampsia [PE], gestational hypertension [GH], fetal growth restriction [FGR], and miscarriage).
In the study group the following data were also collected: age at SS diagnosis, time between diagnosis and pregnancy, laboratory criteria for antiphospholipid syndrome (APS), immunotherapy regimens and other medication used during pregnancy. Baseline laboratory data included antinuclear (ANA), anti-Ro/SSA, anti-La/SSB, and antiphospholipid (aPL) antibody (lupus anticoagulant, anti-cardiolipin, and anti-β2-glycoprotein antibodies) tests.
The course of the disease during pregnancy was not detailed because we did not have a standardized tool to retrospectively homogenize these data.
Miscarriage was defined as the involuntary loss of a fetus before the 20th week of gestation, stillbirth as fetal death after the 20th week of pregnancy. Preterm birth was a live birth before the 37th week of gestation. Fetal growth restriction was defined according to the American College of Obstetricians and Gynecologists Committee (ACOG) criteria (2019) [10].
Preeclampsia and GH were defined according to the ACOG criteria [11]. Preeclampsia was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg on two occasions at least 4 hours apart after the 20th week of gestation in a woman with previously normal blood pressure and proteinuria (defined as urinary excretion of 300 mg or more in a 24-hour urine collection or a protein/creatinine ratio of 0.3 mg/dl or more or a dipstick Reading of 2+).
Otherwise, in the absence of proteinuria, PE was defined as new-onset hypertension with new-onset of target organ damage (platelet count less than 100 × 103/l; serum creatinine concentration greater than 1.1 mg/dl or doubling of the serum creatinine concentration in the absence of other renal diseases; elevated blood concentrations of liver transaminases to twice the upper limit of normal concentration; severe persistent right upper quadrant or epigastric pain not accounted for by alternative diagnoses; pulmonary edema; new-onset headache unresponsive to acetaminophen and not accounted for by alternative diagnoses; or visual disturbances) [11].
The American College of Obstetricians and Gynecologists Committee defines GH as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or both, on two occasions at least 4 hours apart after 20th week of gestation, in a woman with previously normal blood pressure in the absence of proteinuria or organ dysfunction, and that returns to normal in the postpartum period.
Obstetrics and Internal Medicine Management Protocol
Whenever possible a preconception consultation was performed. Pregnancy follow-up consists of regular obstetric and internal medicine appointments. The main objective is an early diagnosis of hypertensive disorders of pregnancy, miscarriage, FGR, CHB, and spontaneous preterm delivery (PTD). Serial monitoring of hematological, and immunological parameters was performed. Fetal regular sonographic evaluation with Doppler and fetal echocardiography was also implemented. The main goals were early diagnosis and early treatment of incomplete CHB.
Low-dose acetylsalicylic acid (ASA) was started, if not already prescribed, as soon as pregnancy was documented, according to the institution’s protocol. Low molecular weight heparin (LMWH) was prescribed in all patients with risk factors for thromboembolic disease.
In the SS group, a delivery is programmed by 39 weeks of pregnancy. All patients were reevaluated in the first weeks postpartum.
The control group was managed according to the Portuguese recommendations for low-risk pregnancies [12]. Serial laboratory monitoring was performed and fetal evaluation consisted of one ultrasound scan in each trimester.
Statistical analysis
Descriptive data analysis was performed. Categorical variables are presented as frequencies and percentages and continuous variables as means and standard deviations. The χ2 test and Fisher’s exact test were used to establish associations between categorical variables. The independent samples t-test was applied for continuous variables.
The denominator used in the analyses of all maternal outcomes, stillbirths, and live births was all reported pregnancies. For all other maternal-fetal outcomes, the denominator was all live births. The IBM SPSS Statistics version 27.0.1 was used to perform the analysis all reported p-values are two-tailed, with a p-value of 0.05 indicating statistical significance.
Results
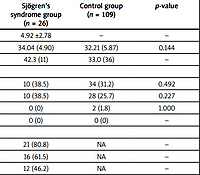
A total of 26 pregnancies with SS were analyzed. At the time of diagnosis, the mean maternal age was 31.46 ±6.77 years. All pregnancies occurred after the diagnosis of SS, with a mean time between diagnosis and conception of 4.92 ±2.78 years. Mean age at delivery did not differ significantly between groups (34.04 years in the SS group vs. 32.21 years in the CG, p = 0.144).
Other autoimmune comorbidities were common in the SS group, including SLE (23.1%) and other autoimmune disorders (46.2%) (p < 0.01). Mixed connective tissue disease (MCTD) was present in 3.8% of SS patients (p = 0.193), and no cases of APS were previously diagnosed. In controls, no women had SLE, APS, or MCTD, and 1.8% had other autoimmune disorders.
In the SS group, the incidence of ANA, anti-Ro/SSA, and anti-La/SSB antibodies positivity was 80.8%, 61.5%, and 46.2%, respectively. Among these female women, 38.5% had primary SS, and 61.5% had secondary SS. Baseline clinical characteristics of pregnant women are shown in Table I.
Table I
Baseline clinical characteristics of pregnant women
In the SS group, the miscarriage rate was 19.2%. In the control group, the incidence of this outcome was 1.8%, p < 0.01. These data correspond to an OR of 12.7 (CI: 2.31–70.10, p < 0.01). No case of stillbirth was registered in either group. Fetal growth restriction incidence was higher in the SS group; the difference was not statistically significant (9.5% vs. 0.9%, p = 0.054). Preeclampsia occurred in 1 (0.9%) case of the controls and no cases were observed in the SS group. No cases of GH were observed in both groups.
The mean weeks at delivery were not significantly different between the SS and control groups (38.05 ±1.27 weeks vs. 38.65 ±1.82 weeks, p = 0.171). Preterm delivery (7.7% in the SS group vs. 5.5% in the CG, p = 0.346) and mean neonatal birth weight (2998.16 g in the SS group vs. 3155.79 g in the CG, p = 0.178) were also not significantly different. Cesarean section in the SS group was performed for six pregnancies and did not differ significantly from the CG (28.6% vs. 26.2%, p = 0.820). There were no neonatal deaths in both groups. Apgar scores at the 1st and 5th minutes were similar in both groups.
The newborn admission rate in the NICU was significantly higher in the SS group (23.8% vs. 0%, p < 0.01). In women with SS three pregnancies were complicated by CHB (14.3% vs. 0%, p = 0.015), two of them with an intrauterine fetal AV block II and one with a complete AV block.
In all cases, the diagnosis was performed in the 2nd trimester of pregnancy and 6 mg/day of betamethasone was administered. None of the fetuses diagnosed with AV block regressed. These cases had a preterm delivery. One neonate required pacemaker implantation during the first month of life. No other congenital anomalies were observed.
During pregnancy, treatment was administered in the majority of women diagnosed with SS. Hydroxychloroquine (HCQ) was used in 57.7%, ASA in 80.8% prophylactically, and azathioprine in 15.4%. Eight (30.8%) patients received LMWH prophylactically.
Pregnancy and neonatal outcomes in women with SS and controls are shown in Table II.
Table II
Pregnancy and neonatal outcomes in women with Sjögren’s syndrome and controls
Discussion
Autoimmune diseases can influence obstetrics outcomes. In autoimmune diseases such as SLE and AR, the impact of the disease on fetal and pregnancy outcomes has been well established [5, 13, 14]. On the other hand, the influence of SS in pregnant women is still limited and conflicting.
To the best of our knowledge, this is the first case-control study involving a Portuguese pregnant group with SS disease. Our results highlight that gestation in SS patients is associated with an increased incidence of adverse obstetric and neonatal outcomes, particularly miscarriage, admission to a NICU, and CHB.
Our study showed a higher incidence of miscarriage in SS patients than controls, which is consistent with previous studies [6, 8, 15, 16]. The increased rate of miscarriage can be explained by immunological disturbance, which can interfere with the physiological development of the placenta [7, 8]. Contrarily, a study by Hussein et al. [4] did not confirm a high number of miscarriages in SS women. The lower rate observed is probably due to the study design and the small number of pregnancies (n = 16).
In our study, aPL antibodies without fulfilling the criteria for APS were present in 15.4% of women with SS. Three of the four women with positive aPL antibodies had a miscarriage. However, the small number of women (n = 4) limits our analysis of the potential impact of aPL antibodies. A retrospective cohort study by Luo et al. [17] reported that positive anti-Ro/SSA in addition to APS was associated with fetal loss. Nevertheless, other studies did not find any association between miscarriage and the presence of anti-Ro/SSA, anti-La/SSB, or aPL antibodies [5, 9].
A recent meta-analysis of seven studies by Upala et al. [6] found that SS is associated with an increased risk of fetal loss. In our results, no cases of stillbirth were reported in both groups. However, Upala et al. [6] also reported that several of the studies in their meta-analysis date back to the 1990s, and these more recent publications did not find any differences in the risk of fetal loss in SS patients. They suggest that this discrepancy between the studies may be due to older classification criteria and limited understanding of SS at the time of the first studies.
In our study, the difference in FGR rate between SS patients and controls was not statistically significant. Our results were similar to those of the study by De Carolis et al. [8]. On the other hand, Elliot et al. [3] reported a higher incidence of FGR in women with SS and suggested that it could be related to placental insufficiency. They argued that the discrepancy between their results and other studies may be attributed to the larger sample size of their study. In the present study, all women with FGR had concomitant LES. Previously published studies report a significantly higher incidence of FGR in SLE patients than in the CG [14, 18, 19].
The incidence of preterm delivery was significantly higher in women with SS [3, 8, 15]. In our study, the preterm delivery rate was similar between groups. This may be explained by the low incidence of FGR and hypertensive disorders. In our study, a high percentage of patients were medicated with HCQ. Hydroxychloroquine treatment might have a positive effect on the perinatal outcomes [20], which may also could support our findings.
The incidence of cesarean section in the study group was similar to the control group. Ballester et al. [15] reported similar results. However, it was discordant with previously published studies, reporting an increased risk of cesarean delivery in women with SS [3, 4]. This could be explained by the greater incidence of FGR in those studies.
As expected, and following the previously published literature, admission to the NICU was higher in the study group (23.8%). It is due to the higher incidence of CHB. Neonatal congenital heart block is the most significant complication which may affect the offspring of women with a diagnosis of SS [3]. It is one of the different manifestations referred to as neonatal lupus [21].
In our study, CHB was related to the presence of anti-Ro/SSA and/or anti-La/SSB antibodies. These antibodies can cross the placental circulation and cause damage in the cardiac fetal tissue affecting the conduction system, leading to AV block after causing inflammation and fibrosis [8, 21, 22]. There are three AV block degrees: first, second (incomplete), and third (complete) [22], the last one being irreversible. The CHB has an associated mortality rate of 30% [5, 15, 22]. The reported prevalence rate of CHB in the offspring of anti-Ro/SSA and anti-La/SSB positive women is approximately 2% and 3%, respectively [5].
There is no definitive and effective treatment for fetal CHB. When CHB is diagnosed during pregnancy, maternal treatment with fluorinated steroids (as dexamethasone or betamethasone) is recommended in cases of first or second degrees of AV block [7, 22], as we observed in our study. The use of steroids may reduce the antibody-mediated inflammatory damage of nodal tissue and prevent progression to complete AV block or reverse incomplete AV block [7, 8, 22].
Some studies report alternative therapies: plasmapheresis, intravenous immunoglobulins, and sympathomimetics [5, 7, 8]. A complete AV block is irreversible and a potentially lethal condition, and most (two-thirds of cases) affected children will require permanent pacemaker implantation [15, 23]. In our study, one neonate required pacemaker implantation after birth.
In this study, despite preventive treatment with HCQ, three fetuses developed second and third-degree CHB. Betamethasone was added from CHB detection until delivery. The limited number of neonatal lupus cases limits our analysis. Some studies reported that HCQ could reduce the risk of recurrence of neonatal lupus, especially cardiac involvement [17]. However, further studies are needed to understand the effect of HCQ on the development of neonatal lupus.
Study limitations
Our research has some limitations associated with its retrospective nature and therefore has several weaknesses, which include inherent biases, including selection bias and information bias. On the other hand, the control group comprised a population of healthy pregnant women for comparing the presence of complications. The generalizability of this study should be interpreted with caution as it was conducted in a single tertiary university hospital in Portugal.
Conclusions
Women with SS, like with other autoimmune diseases, had a higher risk of adverse pregnancy outcomes in comparison with healthy mothers. The study shows a higher incidence of miscarriage, admission to the NICU and CHB events than in healthy pregnacy women group. In women with SS is essencial a proper monitoring and eductaion patients with anti-Ro/SSA and/or anti-La/SSB antibodies and risk of CHB and neonatal lupus.
Pre-pregnancy planning, anticipating complications monitoring by fetal echocardiography and pregnancy follow-up are essential to enhance pregnancy outcomes. Successful pregnancy in women with SS is possible with a care of multidisciplinary team in the centers of reference. The use of HCQ may also help to improve the obstetric outcomes in SS patients.
This study represents the first comparison of maternal and fetal outcomes between pregnant women with and without SS in a Portuguese cohort.
In the future, a multicenter prospective study to better clarify the impact of SS on obstetric outcomes, and likewise the impact of HCQ on pregnancy outcomes, should be performed.