Introduction
The global incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has surpassed 771 million cases, resulting in almost 7 million deaths as of October 3, 2023 [1]. Over the course of the three previous years, the SARS-CoV-2 virus has exerted an exceptional and profound impact on individuals worldwide, particularly those who are afflicted by autoimmune rheumatic diseases (AIRDs).
Patients with AIRDs in most cases are more likely to be hospitalized with SARS-CoV-2 [2, 3]. The severity of the underlying disease and use of immunosuppressive medication, disruption of immunoregulation, and a comorbid background, all contribute to infection vulnerability [4].
Vaccination is acknowledged by experts as a highly effective approach in combatting the COVID-19 pandemic. Considering the lack of statistically significant data showing increased survival in COVID-19 with antiviral drugs, this circumstance appears to be particularly relevant [5].
The world community acknowledges the significance of administering COVID-19 vaccinations in patients with autoimmune disorders. The Advisory Committee on Immunization Practices emphasizes the priority of AIRD patients to receive vaccination over the general population of the same age and sex [6]. As patients with AIRDs are at risk for severe COVID-19 and other infectious diseases, vaccination should be a priority for providing highly qualified medical care in rheumatology [7].
According to data from a physician-reported registry [8], COVID-19 immunization has been shown to be safe and tolerable in people with AIRDs. A series of large studies demonstrated the safety of immunization against SARS-CoV-2 in patients with rheumatoid arthritis (RA) [9], systemic sclerosis (SSc) [10], systemic lupus erythematosus (SLE) [11], and inflammatory myositis (IIM) [12]. Although vaccination apprehension appears to be a significant issue in the general population, rheumatological patients are not an exception [13].
Regarding the scarcity of available evidence about the safety, effectiveness, and hesitancy of COVID-19 vaccines in AIRDs patients, particularly from Asian countries, we conducted this survey to investigate actual data. The study objectives were to assess the vaccination status, adverse effects (AEs), breakthrough infections and flares of underlying rheumatic disease. We studied post-vaccination periods during all pandemic waves. According to official data, Kazakhstan had the first case of COVID-19 infection in March 2020 with considerable increases in cases from April 2020. The number of Kazakhstan citizens vaccinated both with the first and the second components was 1.871 and 0.778 million by May 18, 2021 [14]. This is 9% of the country population. The Kazakhstan-made vaccine QazCovid-In (QazVac) was implemented after the third phase of clinical trials, since April 26, 2021 [15].
In the Republic of Kazakhstan, as of 2023, RA and ankylosing spondylitis (AS) are the most prevalent AIRDs (6,601 and 4,468 cases), followed by SLE (n = 4,071) and SSc (n = 613) [16]. The SARS-CoV-2 infection prophylaxis is important in AIRDs, which confirmed EULA/ACR recommendations. However, in different countries and different populations vaccination rates vary and different post-vaccination phenomena have been recorded.
The aim of this study was to assess, based on a questionnaire, the occurrence of specific post-vaccination symptoms in a group of patients with diagnosed rheumatic diseases.
Material and methods
This cross-sectional interview-based study was conducted in Astana city policlinics No. 4, No. 7, No. 8. The research was carried out from April to July 2023. Adult patients over the age of 18 with a confirmed diagnosis of RA, SLE, SSc and AS were enrolled in the study. The survey was conducted using a structured questionnaire. Patients who answered the whole questionnaire and received at least one dose of a COVID-19 vaccination were identified. We included four more frequent occurrence rheumatic diseases in the study, which include RA, SLE, SSc and AS. The study excluded respondents with other AIRDs, who did not receive any dose of COVID-19 vaccine prior to completing the survey and did not fully complete the questionnaire. A comprehensive patient-self-reporting electronic survey was developed in Kazakh language and then translated into Russian as and when required. The questionnaire consisted of a total 18 questions: 2 questions about personal data, 7 questions pertaining to the clinical characteristics of AIRD and comorbidity, 3 questions based on COVID-19 infection and 6 regarding vaccination against SARS-CoV-2. Details on COVID-19 vaccination, demographics, AIRD diagnosis and treatment, current symptom status, 7-day short-term post-vaccination AEs and patient-reported outcome measures were also included.
The disease activity was initially evaluated by a question about the status of autoimmunity prior to the first dose of the COVID-19 vaccine. The response “My disease was stable/inactive” was considered to indicate a remission. The respondents who answered “My disease was active/deteriorating” were classified as having an active disease.
Adverse effects were classified as minor and major with or without hospitalization. Serious vaccination-related adverse events which required emergency medical attention were categorized as major AEs. These reactions included anaphylaxis, respiratory distress, laryngeal edema, severe dermatological manifestations and hypertensive crisis. Minor AEs comprised myalgia, body aches, fever, chills, nausea and vomiting, stomach pain, exhaustion, diarrhea, rapid pulse or palpitations, increase in blood pressure, fainting, dizziness, and chest pain.
Statistical analysis
Frequency analysis was used to describe categorical data. Average value and standard deviation were used for quantitative data. The normality of the distribution was evaluated using the Shapiro-Wilk test. Fisher’s exact test was used to analyze binary categorical variables; in other cases to analyze categorical variables the chi-square test was used. Student’s t test was used for comparison of quantitative data for independent samples. A result of p < 0.05 was considered statistically significant in all tests. Statistical analyses were performed using IBM SPSS software (version 19).
Results
Patients’ characteristics
In this study, we investigated in total 193 patients with AIRDs, who were observed in policlinics No. 4, No. 7, No. 8 of Astana city. After exclusion criteria application, patients with other AIRDs and unvaccinated individuals were not considered. Finally, 62 respondents participated in the analysis. The majority of them were female (69.4%, median aged 50.3 ±12.9 years). The female to male ratio was ~ 2.3 : 1. Asian was the most common ethnicity (87%), followed by European (13%). Rheumatoid arthritis (35.5%, n = 22) followed by AS (29%, n = 18) were the common AIRDs in our study population. The most common observed comorbidities were arterial hypertension (30.6%, n = 19) and chronic obstructive pulmonary disease (14.5%, n = 9).
Genetic-engineered biological agents (GEBA) were the most common treatment option in our cohort: any biological therapy was used by 15 (24.2%) patients. The most frequently used disease-modifying antirheumatic drugs (DMARDs) were leflunomide (16.1%, n = 10;) and methotrexate (14.5%, n = 14). Glucocorticosteroid (GC) therapy was received by 30 (48.4%) patients. Other characteristics of the background therapy are provided in Table I.
Table I
Distribution of vaccinated patients depending on AIRD therapy
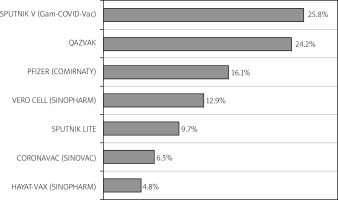
Among 193 patients, 62 (32.1%) were vaccinated. All patients received at least one dose of the COVID-19 vaccine, 36 (58.1%) of whom had two and 16 (25.8%) three doses of vaccine. Sputnik V, a vector vaccine based on an adenovirus (Gam-COVID-Vac), was the most common (25.8%, n = 16) vaccine received, followed by QazVac, an inactivated whole-virion vaccine against COVID-19 (QazCovid-in) (24.2%, n = 15). Figure 1 illustrates the percentage of vaccinated patients distributed according to the vaccine type.
There were 131 patients who declined to receive the vaccine, and more than half of them (68%) were concerned about autoimmune status and probable worsening of the disease. Only 15 (24.1%) of vaccinated patients discussed their decision with a rheumatologist before getting the vaccine.
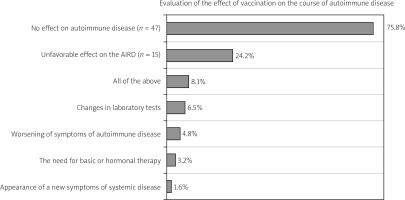
We registered patient-reported flares in 16 (24.2%) cases, where patients assessed the course of the systemic disease as long-term deterioration after using a vaccine. At the same time, 4 (6.5%) patients noted changes in laboratory tests, 3 (4.8%) noted an aggravation of symptoms of autoimmune disease, and only 1 (1.6%) patient reported the appearance of previously unregistered new symptoms in the post-vaccination period. However, none of the patients required hospitalization due to an exacerbation of the AIRD within 21 days after vaccination. Patients’ opinions on the vaccination effect on autoimmune disease are shown in Figure 2.
Post-COVID-19 vaccination-associated adverse effects
Any vaccine-related AEs were reported in 42 (66.7%) patients; all of them were minor. The most common AE was injection site pain, reported by 26 (41.9%) patients. Other minor AEs indicated were muscle and joint pain in 22 (35.5%), body pain in 15 (24.2%), fever in 12 (19.4%), chills in 9 (14.5%), headache in 7 (11.3%), fatigue in 6 (9.7%), and tachycardia in 5 (8.1%). Only one patient (1.6%) reported any major vaccine-related adverse effects. Post-COVID-19 vaccination-related AEs among patients with self-reported active and inactive disease are presented in Table II.
Table II
Comparison of the most frequent vaccine-induced side effects depending on AIRD activity
Among 62 patients, 34 (55%) had remission; 5 and 17 (8.06% and 28.3%) had high and moderate disease activity, respectively. Additionally, 6 patients (9.6%) answered “I don’t remember” or “I can’t estimate the disease activity prior to vaccination”. Statistically significant differences were found in patients with headaches in minimal activity of AIRD. It was registered in 7 (20.6%) cases of remission, but was not identified in moderate and high disease activity (Fisher’s test, p = 0.035).
We evaluated vaccination-related AEs among patients based on the background immunosuppression. Overall, the frequency of vaccination-related AEs was similar between the vaccine subgroups. No statistically significant differences were found (χ2 Pearson). We also intended to analyze the influence of age (p = 0.066), sex (p = 0.377), and comorbidities (hypertension [p = 0.872], diabetes [p = 0.671], chronic obstructive pulmonary disease [p = 0.133], thyroid pathology [p = 0.671], obesity [p = 0.705]) with vaccination-related AEs. However, none of them had a statistically significant relationship (Student’s t-test for independent samples and Fisher’s test for binary categorical variables). Statistically significant differences were found in patients who noted AEs and subsequent exacerbation of systemic disease. The vaccination provoked an exacerbation of AIRD in 8 (19%) patients with AEs. There were no cases of exacerbations in patients without AEs (χ2 = 7.23, df = 2, p = 0.026). Among patients without AEs, 16 (80%) people reported no effect of vaccination on the course of autoimmune disease. Only 24 (57.1%) patients with AEs denied the vaccination effect on the course of autoimmune disease. No significant differences were found for other values. The association of vaccine-induced AEs and the AIRD status prior to vaccination is shown in Table III.
Table III
Association of vaccine-induced side effects and AIRD status
An infection occurring more than 2 weeks after receiving a first or second dose of the COVID-19 vaccine was considered as breakthrough infection. A total of 12 (19.4%) patients were infected with COVID-19 during 180 days after vaccination, of whom 8 (12.9%) received a single dose and 4 (6.5%) were vaccinated completely. None of the patients had a severe course of the infection with requirement of hospitalization, oxygen therapy and no-invasive or invasive ventilation.
Discussion
It is important to understand that patients with AIRDs are in “uneven” conditions. For example, a low-activity RA patient receiving hydroxychloroquine is likely to have a lower risk of developing COVID-19 and adverse outcomes compared to an active vasculitis patient receiving intravenous cyclophosphamide/rituximab with high doses of glucocorticoids [17]. Retrospective cohort analysis from the U.S. [18] demonstrated that individuals with diagnosed AIRDs had a higher susceptibility to breakthrough infections. Furthermore, this risk was found to potentially differ depending on the kind of underlying rheumatic disease. Rheumatoid arthritis per se and antirheumatic drugs are able to suppress the immune response from vaccines in different extents [19]. In 2023, Li et al. [20] published the results of a study which examined risks and outcomes for SARS-CoV-2 before and after COVID-19 vaccination. The results confirmed that vaccinated patients with RA are still at elevated risks of COVID-19 infection and hospitalization compared to the general population after receiving COVID-19 vaccines. Vaccination against COVID-19 can provide various degrees of protection of rheumatological patients [18].
Importantly, while rheumatological patients under immunomodulatory treatment exhibit reduced seroconversion rates after SARS-CoV-2 vaccination, the neutralizing capacity of anti-SARS-CoV-2 antibodies is the same in people with and without AIRDs [21]. Breakthrough infections were observed in 7.4% (n = 47) of cases in the study by Ahmed et al. [22], who examined the post-vaccination anti-body titers in patients with autoimmune rheumatic diseases. They concluded that breakthrough infections were significantly correlated with a lack of or inadequate antibody response. Antirheumatic drugs may also adversely affect the post-vaccination response and increase the risk of developing a breakthrough COVID-19 infection in vaccinated patients. Patients receiving rituximab (RTX) were also at particular risk [23]. According to the data obtained from the COVAX registry [8], the occurrence of breakthrough infections in patients with AIRDs who received complete vaccination was 0.7%. The result was lower than the rate observed in fully vaccinated people without immune disorders (1.1%) and there was no statistically significant relationship between breakthrough infections and the vaccine type. Similar data were obtained in another cross-sectional study, where the prevalence of breakthrough infections was found to be 8.4% among vaccinated and 16.4% among non-vaccinated individuals [24].
In our study, breakthrough infections were observed in 12 (19.4%) patients, 8 (12.9%) of whom received a single dose and 4 (6.5%) were vaccinated completely. The incidence did not differ statistically significantly between the used vaccines types. None of the patients was hospitalized, required supplemental oxygen or mechanical ventilation, or died.
According to the data [25], the incidence of autoimmune disease activation after COVID-19 vaccination ranges from 5% to 7% and has no statistically significant associations with vaccine type or antirheumatic therapy. In our cohort autoimmune disease flares following vaccination were seen in 24% of cases. All of them were managed conservatively. The previously discussed COVAX registry reported flares in 4.4% [8]. Background antirheumatic therapy had no significant effect on the frequency of post-vaccination exacerbations and had a possible association between the disease activity and severity of flares. Patients with high disease activity had more exacerbations (5.2%) than patients in remission or low disease activity (4.8%). In the COVID-19 Global Rheumatology Alliance survey, vaccine-related rheumatic disease flares requiring treatment modification were registered in 4.6% [26].
Other studies found no evidence of rheumatic disease flares after vaccination. The aforementioned symptoms were likely categorized as post-vaccination adverse effects that did not require significant modifications in the treatment options of the systemic disease [27–29]. Some studies indicated post-vaccination increases in inflammatory activity (erythrocyte sedimentation rate, SR protein) with subsequent normalization without any serious consequences [30, 31].
There exists significant scientific interest in the discussion concerning the relationship between COVID-19 vaccines and immuno-mediated adverse events. In data obtained from a metaanalysis [31], 460 cases with various post-COVID vaccination complications were registered. The majority of cases (51.5%, n = 237) consisted of thrombosis ranging from cerebral venous thrombosis and thrombosis thrombocytopenic purpura to pulmonary embolism. Other cardiac and vascular complications (14.2%, n = 66) included intracranial hemorrhage, myocarditis, disseminated intravascular coagulopathy, idiopathic thrombocytopenia and myocardial infarction. Other AEs included neurological, dermatological, hematological, renal and ocular complications. Notably, vaccine-specific AEs were observed in relation to a specific vaccine. The most prevalent association is AstraZeneca/thrombophilia, which were registered in middle-aged males. mRNA vaccines were associated with idiopathic thrombocytopenia, myocarditis and glomerulopathy, while inactivated vaccines were associated with ocular adverse events. It should be emphasized that the AstraZeneca vaccine induces an immune response by nCoV-19 spike protein, while the mRNA-vaccine triggers an antibody response with a lipid-nanoparticle-encapsulated mRNA. Such variability in the immune response initiation mechanisms may be responsible for the various adverse profile associated with each vaccine.
In the analysis of the COVAX registry [8] with 121 participants from 30 countries, 13 (37%) cases of AEs were identified; 0.5% of them were serious. The majority of patients (70%) received Pfizer/BioNTech, 17% were vaccinated with AstraZeneca/Oxford and 8% with mRNA Moderna vaccines. Most of them resolved without serious consequences. Undesirable effects were not found to be statistically significantly associated with any vaccine. Notably, following administration of the Johnson & Johnson and AstraZeneca vaccines, there were no reports of vaccine-induced immune thrombocytopenia, an exceedingly rare complication observed in the general population after receiving the AstraZeneca and Johnson & Johnson vaccines.
In our study 66.7% of all vaccinated patients reported vaccine-associated AEs. These adverse effects were mild and none required hospitalization. The most frequent side effects were injection site pain (41.9%), muscle and joint pain (35.5%), body pain (24.2%) and fever (19.4%), which were similar to other surveys [32].
In the COVAD cohort [10, 33], the majority of AEs were not serious and were similar for active and inactive SSc and SLE. The IIM patients with active disease, who had higher incidence of AEs, were excluded [34]. The frequency of vaccine-related AEs and hospitalization were similar irrespective of background immunosuppression for all studies.
During widespread immunization programs, the issue of vaccination reluctance among people with AIRDs has gained considerable significance [12]. The study from the Global Rheumatology Alliance Vaccine Survey [35] demonstrated that among 7,005 patients with AIRDs from 102 countries, 21% reported vaccine hesitancy as the most common reason for being concerned about vaccine side-effects. Other reluctant respondents expressed worries about vaccine safety, flares of underlying disease and the rapid research and clinical application of the vaccine. Even patients who expressed reluctance revealed various degrees of hesitancy, including two-thirds noting that their willingness may potentially be enhanced. It should be emphasized that rheumatologist endorsement was generally a crucial factor that increased vaccination compliance [13]. In our study, only 24% of patients consulted their rheumatologist prior to receiving the vaccine, indicating that the role of the doctor in vaccination decision-making may have been undervalued. It is important to underline, that international rheumatologic scientific societies support the position about benefits of vaccination, which considerably outweigh the potential harm associated with the development of undesirable effects [36–40].
Study limitations
The study has several limitations inherent to the small sample size and the imperfect design with a lack of a control group. An immunocompetent control group could have provided a further perspective to the subject of study. The retrospective data collected in a self-reported electronic survey cannot be confirmed by medical records and is affected by recall bias. The online patient survey approach neglecting a doctor’s examination should be taken into account in future studies as a “weak point” in collecting reliable data on the incidence of AIRD exacerbations subsequent to COVID-19 immunization. Additionally, the severity of AEs was not quantified in our study and needs to be further investigated.
The differentiation of active and inactive disease that relies on patients’ subjective self-reports of their disease activity would make it difficult to interpret the data gathered clearly due to significant differences in the patients’ interpretation of exacerbations and the long post-vaccination period of observation. The cohort contained a conveniently selected sample excluding diseased patients and participants without access to the internet or mobile devices, with a negative socioeconomic position, and severe physical and mental disabilities.
Conclusions
Our results contribute to the growing body of evidence showing that patients with compromised immune systems may receive vaccinations safely with no major vaccine-related AEs. No major differences in AEs and breakthrough infections were found between the vaccine subgroups. Although vaccination reduces the risk of infection and the severity of the COVID-19 course, there are still questions about the safety of vaccination against SARS-CoV-2. The decision to vaccinate against SARS-CoV-2 should be individualized and based on the mutual compliance between doctor and patient. The current epidemic situation, the activity of AIRD and background immunosuppression have to be taken into account. The latest scientific and clinical research data should be used for discussion with the patient concerning the benefits, risks, advantages and negative aspects of vaccinations.
Unfortunately, the data on vaccination against SARS-CoV-2 of patients with AIRDs in the Republic of Kazakhstan are notably limited. The realization of region-specific recommendations and studies has the potential for reducing vaccine reluctance among the population, consequently improving the achievement of vaccinations purposes. The authors hope that the results will contribute to confidence in the safety of vaccination against SARS-CoV-2 among both colleagues and patients in the field of rheumatology.