Introduction
Systemic sclerosis (SSc) is an autoimmune connective tissue disease, characterized by progressive fibrosis and vasculopathy. It typically affects the skin and other internal organs. Cardiac involvement, with a clinical prevalence of 15% to 35%, appears to be one of the major causes of mortality. According to the European Alliance of Associations for Rheumatology (EULAR) Scleroderma Trials and Research (EUSTAR), among the most common causes of mortality in SSc are interstitial lung disease (ILD; 35%), pulmonary atrial hypertension (PAH, 26%), and cardiac causes (26%), mainly heart failure and arrhythmias [1].
Cardiac diseases associated with SSc are myocarditis, myocardial fibrosis, conduction system abnormalities, coronary artery disease, pericardial disease, and valvular disease. Although cardiac involvement has traditionally been associated with diffuse SSc (dSSc), there has been increasing evidence that patients with limited SSc (lSSc) may also have significant cardiac abnormalities.
The pathophysiological mechanism in the early stage of the disease is a reversible spasm of capillaries similar to Raynaud’s phenomenon observed on the fingers. In the early phase of the disease, patients usually have no clinical symptoms. With the progression of the disease, structural abnormalities of the small coronary arteries occur.
This results in the deterioration of myocardial microcirculation, inflammation, and myocardial fibrosis. Diastolic and systolic dysfunction is observed. Both the right and left ventricles can be affected by fibrosis, causing impaired relaxation and contractility of the heart. Moreover, atherosclerosis and coronary artery disease may occur apart from microcirculation disorders. Right ventricular failure as a consequence of PAH remains a separate issue [2–6].
In our study, we aimed to compare patients with and without heart involvement in the course of SSc.
Material and methods
Electronic medical records of patients treated between January 2021 and August 2022 in the Department of Rheumatology were searched for the diagnosis of SSc (ICD-10 code M47). We diagnosed primary heart involvement in patients with cardiovascular symptoms and abnormalities in Holter ECG (electrocardiogram), echocardiography, and blood tests that could not be explained by coexisting cardiovascular diseases such as hypertension, hyperlipidemia, or coronary artery disease.
Patients with diagnosed PAH (confirmed by right heart catheterization) were enrolled in the control group. The clinical characteristics of patients with and without primary heart involvement in the course of SSc were compared.
The following laboratory data was collected: C-reactive protein (N < 5 mg/dl), N-terminal pro-brain natriuretic peptide (NT-proBNP; N < 100 pg/ml), troponin T (N < 14 ng/l) and creatinine kinase (CK; N < 192 U/l) levels. Patients with elevated CK were diagnosed with myositis in the course of SSc. Skin involvement was assessed in all patients with the modified Rodnan skin score (mRSS) [7].
Capillaroscopy was performed in all patients using a Dino-Lite CapillaryScope; the type of microangiopathy was assessed according to Cutolo et al. [8] as early, active, and late phase. The microangiopathy evolution score (MES) was assessed as the sum of three scores: loss of capillaries, disorganization of the microvascular array, and capillary ramifications. All patients underwent high-resolution computed tomography (HRCT) with the assessment of the type of ILD using a 64-slice CT scanner.
In some patients, pulmonary function tests were performed, and diffusing capacity of the lungs for carbon monoxide (DLCO) was calculated using the Neas prediction equation and corrected for hemoglobin. Gastrointestinal tract involvement was diagnosed mainly based on esophageal achalasia described in chest computed tomography (CT) or esophageal X-ray with barite. Data on immunosuppressive and rheological treatment were also collected.
All patients with heart involvement underwent transthoracic echocardiography examination (Philips EPIQ 7G device), which assessed left ventricular diastolic diameter, left ventricular systolic diameter, intraventricular thickness, left ventricular ejection fraction (LVEF), early diastolic velocity (E’), early velocity (E), peak velocity at the time of atrial contraction (A), right ventricular diastolic diameter, and right ventricular systolic pressure (RVSP, mm Hg).
Values of Eʹ < 7 cm/s, E/Eʹ > 14, and E/A > 2 are considered abnormal and indicate diastolic failure [9]. Mean pulmonary arterial pressure (mPAP ) was calculated according to the formula: mPAP = 0.61 × RVSP + 2 mm Hg and the value mPAP > 25 mm Hg indicates PAH [10].
Pericardial effusion was measured as the largest distance between the parietal and visceral pericardium at the diastole behind the left ventricular posterior wall. Patients with heart involvement also had 24-hour Holter ECG, searching for ventricular arrhythmias (tachycardia and ventricular extrasystoles), supraventricular arrhythmias (atrial flutter and extrasystoles), and the conduction disorder atrioventricular block. Only two patients underwent heart MRI (1.5 T).
Statistical analysis
Compliance of the data with the normal distribution was assessed using the Shapiro-Wilk test. The significance of the observed differences between the two groups was assessed using Student’s t-test for variables with a normal distribution and the Mann-Whitney U test for variables without a normal distribution. For categorical variables, Fisher’s exact test (due to tables having values less than 5) was used. Risk factors for cardiac involvement were assessed in a multivariate analysis using logistic regression.
Only patients without missing data were included in the multivariate analysis. Statistical significance was set at p < 0.05. Statistical analysis was performed using Statistica 13.3 software (StatSoft Polska, Kraków, Poland).
Results
Out of 36 (25 females, 11 males) patients with SSc, 7 patients were diagnosed with heart disease in the course of SSc. Characteristics of SSc patients are presented in Table I.
Table I
Characteristics of patients with systemic sclerosis with (heart involvement group) and without heart involvement (control group)
Risk factors for developing heart disease in the course of SSc were male gender (p = 0.018), diffuse type of SSc (p = 0.03), higher values of mRSS (p < 0.001), gastrointestinal tract involvement (p = 0.027), and myositis (p = 0.018). Patients with heart involvement were more frequently treated with cyclophosphamide (CP) than patients without heart involvement (p = 0.05).
Characteristics of cardiac involvement in patients with SSc are presented in Table II.
Table II
Characteristics of cardiac involvement in patients with systemic scleroderma
The major type of cardiac involvement was myocarditis (71%; Fig. 1), and pericarditis was noted in 2 patients (29%; Fig. 2).
Fig. 1
Increased signals of the left ventricle walls on T2-weighted MRI suggesting myocarditis in a patient with systemic sclerosis.
Magnetic resonance imaging shared thanks to Magdalena Marczak from the Department of Radiology National Institute of Cardiology, Poland.
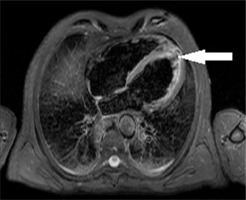
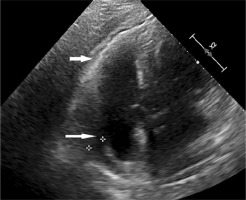
Fig. 2
A fluid with a fibrin layer behind the right atrium and the right ventricle visualized in echocardiography of a patient with systemic sclerosis.
Department of Radiology, National Institute of Geriatrics, Rheumatology and Rehabilitation, Poland.
The majority of patients with heart involvement had elevated troponins (86%) and T-proBNP (71%) concentrations. The most common finding observed in echocardiography was diastolic failure (71%). The most frequent abnormality found in 24-hour Holter ECG was isolated supraventricular extrasystoles (100%).
Discussion
Risk factors of cardiac involvement in systemic sclerosis
In our study, we found that male gender is associated with a severe course of SSc with cardiac involvement, which is consistent with previous studies [11]. We also found that a risk factor for heart involvement is extensive skin sclerosis expressed as high values of mRSS.
Similarly, Domsic et al. [12] demonstrated that severe cardiac involvement was statistically significantly more common in patients with rapid skin thickness progression. However, the relationship between the severe course of SSc and cardiac involvement does not apply to patients with interstitial lung disease – no such association was found in our or previous studies.
According to the literature, type of autoantibodies seems to be a good predictor of heart involvement. Anti-Scl-70 antibodies are associated with a severe course of SSc, including cardiac disease [13].
In our study patients with heart involvement less frequently had anti-centromeric antibodies (which are related to lSSc) and more often had dSSc. What our study failed to show, probably due to the small size of the group, was an association with the pattern of microangiopathy seen in capillaroscopy.
Markusse et al. [14] stated that microangiopathy might be an important cause of organ involvement in SSc independent of autoantibodies, with the late phase of microangiopathy suggesting more severe organ involvement.
Surprisingly, in our study gastrointestinal tract (particularly esophagus) involvement and myositis were associated with a greater risk of heart involvement in SSc, which has not been described in the literature so far.
Types of cardiac involvement in systemic sclerosis
In the present study, the most common type of heart involvement in SSc was myocarditis, which is consistent with the literature [5, 15]. Myocarditis in SSc is associated with high mortality. In a study comparing patients with myocarditis confirmed in an endomyocardial biopsy, the number of patients who died during follow-up due to cardiac complications was significantly higher in SSc patients (50%) compared to myocarditis related to other systemic autoimmune diseases [16]. Early detection of myocarditis allows the initiation of immunosuppressive treatment and prevents cardiac damage [17].
In the later stages, patients may develop myocardial fibrosis, clinically expressed by ventricular diastolic dysfunction, which was the most frequent abnormality found in our study. The pathologic hallmark of myocardial fibrosis is a characteristic patchy, irregular pattern of lesions, which differs from that observed in atherosclerotic coronary artery disease because they do not correspond to the distribution of a single coronary artery [15].
Systolic dysfunction is less common and occurs mainly due to concomitant coronary artery disease or hypertension, and its incidence is reported to be between 11% and 15% [18]. In our study, the frequency of systolic dysfunction was higher (43%).
Another spectrum of SSc cardiac disease is pericardial involvement, which includes acute pericarditis, chronic pericarditis, pericardial fibrosis, and pericardial effusion. It is often asymptomatic and is rarely complicated by cardiac tamponade. In our study, the incidence of symptomatic pericarditis was slightly higher (28.6%) than the prevalence reported in the literature (7% to 20%) [4, 19].
The most frequent cardiac complications of SSc according to EULAR are arrhythmias and conduction defects. Arrhythmia can be caused by a combination of microvascular injury, the development of fibrosis, and autonomic dysfunction. Moreover, myocardial edema is believed to be an important cause of ventricular tachycardia and ventricular fibrillation.
Fibrotic patches can form non-conductive blockages and induce re-entry mechanisms and ectopic automaticity. Furthermore, SSc-related vasculopathy leads to myocardial hypoperfusion, which aggravates electrical dysfunction. The types of arrhythmias are various.
In a study of 53 patients with SSc in 24-hour Holter ECG monitoring, 66% of patients with abnormal features had supraventricular arrhythmias, 40% ventricular premature beats, and 90% ventricular arrhythmias. Malignant ventricular arrhythmias (pulseless ventricular tachycardia and ventricular fibrillation) are the third most common death cause and are responsible for 5% of mortality of SSc patients [20–22].
In a prospective study, 16% and 8% of SSc patients had a left bundle branch block and a first-degree atrioventricular block respectively [23]. In patients with SSc analyzed in our study, the most common finding in 24-hour ECG was supraventricular extrasystoles. Less frequent were isolated ventricular extrasystoles, supraventricular and ventricular tachyarrhythmia, with the most uncommon being conduction defects.
The rarest cardiac involvement in SSc is valvular disease, particularly mitral valve prolapse, mainly asymptomatic. Degenerative aortic valve stenosis is sometimes observed [24]. We found no significant valve defects in our patients.
Screening and monitoring of cardiac involvement in systemic sclerosis
Some patients with SSc-related cardiac involvement experienced clinical symptoms, but usually it is asymptomatic at the beginning of the disease. Screening for cardiac involvement of SSc should include ECG, 24-hour Holter monitoring, echocardiograms, and laboratory tests: troponin-I (Tn-I) and B-type natriuretic peptide (BNP) levels. The most reliable and sensitive imaging technique in diagnosing pathologies associated with heart involvement in SSc is magnetic resonance imaging (MRI).
Echocardiography, readily available in everyday clinical practice, is sufficient for the diagnosis of pericarditis, but it does not fulfill its role in the search for signs of myocarditis or myocardial fibrosis. Compared with echocardiography, MRI provides additional information by visualizing myocardial fibrosis and inflammation. Myocarditis is mainly detected through diffuse increased signals on T2-weighted imaging.
In addition, T1 imaging and calculated extracellular volume can be used to assess myocardial edema, in which elevated T1 values suggest myocarditis. Fibrosis is detected on MRI with delayed hyperenhancement as gadolinium is trapped in the affected tissue and washed away from the healthy myocardium [25, 26].
Hachulla et al. [27] proved that the high incidence of heart abnormalities observed on cardiac MRI (78%) is consistent with the autopsy studies, which showed that approximately 80% of patients with SSc had histological lesions.
Although MRI of the heart is expensive and difficult to access in Poland, only this examination allows myocarditis to be detected at an early stage, before irreversible fibrosis occurs. This is an obvious limitation of our study – only two patients underwent cardiac MRI.
We often have to predict myocarditis using simple biochemical tests such as troponin and NT-proBNP in routine clinical practice. Fortunately, these laboratory tests correlate well with the severity of myocarditis. The majority of patients with cardiac involvement in our study had elevated concentrations of troponins and NT-proBNP.
Sugiyama et al. [28] stated that BNP may be useful as a screening tool for the detection of myocardial abnormalities in SSc patients. Also, Holter ECG should be a routine examination in every patient with SSc and elevated levels of NTproBNP and/or tTn-I.
Bissel et al. [29] reported that patients with significant arrhythmia had higher baseline high-sensitivity troponin I and NTproBNP.
Treatment of cardiac involvement in systemic sclerosis
A separate, difficult issue for clinicians is the management of SSc-related heart involvement. There are no recommendations for the treatment of primary cardiac involvement in SSc based on clinical trials. The European Alliance of Associations for Rheumatology recommendations do not present treatment strategies for SSc-related primary heart disease [30].
According to French recommendations from 2021, management of heart disease in SSc should be consistent with general cardiological guidelines for the treatment of pericarditis, myocarditis, or arrhythmias [31].
However, based on our clinical experience, this approach may be insufficient for patients with SSc with cardiac involvement – the available recommendations were created for patients with coronary artery disease but the causes of cardiac involvement in SSc are microcirculation disorders and inflammation.
In serious arrhythmias, according to the literature, ablation therapy, implantable cardioverter-defibrillator (ICD) therapy, and pacemakers can be used. An ICD should be considered in patients at high risk of sudden death, in secondary prevention, and in primary prevention for patients with a LVEF < 30% (35% if ischemic) and with proven symptomatic ventricular tachyarrhythmia.
For complete heart block and other serious bradyarrhythmias, the treatment of choice is pacemaker implantation. Anti-arrhythmic drugs must be used carefully for SSc patients, always taking into account concomitant conditions. The β-blockers might aggravate the Raynaud phenomenon and cause digital ulcers. Calcium channel blockers such as verapamil are recommended options to treat atrial or intra-nodal tachycardia. Although amiodarone is the most effective anti-arrhythmic drug, it can worsen pulmonary fibrosis [4, 32–34].
For symptomatic pericardial involvement, according to expert opinions from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, the first-line treatment options are nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, low doses of glucocorticosteroids (GCs), and hydroxychloroquine. Nonsteroidal anti-inflammatory drugs and colchicine therapy should be used with careful monitoring of renal function. Second-line options are methotrexate and mycophenolate mofetil (MMF) [35]. In cases of cardiac tamponade, pericardiocentesis and surgical intervention must be considered [4].
For myocarditis, first-line treatment should be MMF or high doses of GCs and standard treatment for heart failure [35]. Based on case reports, high dose GCs should contain methylprednisolone 100–200 mg given intravenously (i.v.) for three days and with the maintenance dose of oral prednisone 0.5 mg/kg p.o. with a gradual reduction [36]. As a second line cyclophosphamide (CP) i.v. or rituximab should be considered.
In our observation patients with cardiac disease were treated more frequently with CP than patients without heart involvement. In addition, a positive effect of hematopoietic stem cell transplant in SSc-related myocarditis was reported [37].
For ischemic cardiopathy, diastolic dysfunction, or rhythm alterations, standard treatment for the specific heart condition is recommended.
Study limitations
Our study has some limitations. The major limitation is the small sample size and the cross-sectional character of the study. Another limitation is the lack of heart MRI imaging, the gold standard for diagnosing heart involvement in SSc, in all patients.
Additionally, our study only deals with primary heart involvement in the course of SSc. We do not discuss the subject of PAH, which is a very broad topic in itself.
Finally, there is no standard definition of heart involvement in SSc, which makes it difficult to interpret and compare studies of heart disease in SSc [38].
Conclusions
In our study, male gender, diffuse type of SSc, extensive skin involvement expressed by higher mRSS, gastrointestinal tract involvement (disturbances in esophageal motility), and myositis were found to be risk factors of primary heart involvement in SSc.
We recommend screening for cardiac complications in these patient groups. Readily accessible screening tests include biochemical tests such as troponins and NT-proBNP. Also, echocardiography and Holter ECG should be routinely performed in all patients with SSc. When available, patients with suspected cardiac involvement should undergo heart MRI.
However, our study only shows a research direction, and there is a need for further research in larger groups of patients to determine the optimal screening. Most importantly, heart disease in SSc still presents a challenge for clinicians, as treatment guidelines for heart disease related to SSc are lacking.




