Introduction
Osteoporosis is the most common bone disorder, and many papers document its high prevalence in rheumatoid arthritis (RA) [1]. The prolonged inflammatory status, mediated by several inflammatory cytokines [2], as well as the reduced activity and the intake of glucocorticosteroids (GCs), may contribute to accelerated bone loss in RA patients.
In this regard, we aimed to assess whether tocilizumab (TCZ), an interleukin 6 (IL-6) inhibitor, may influence bone mineral density (BMD) and bone metabolism in RA.
Material and methods
In this observational, single-center study, we enrolled patients fulfilling 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) criteria for RA [3] and started treatment with TCZ 8 mg/kg intravenously every 4 weeks.
All patients underwent dual energy X-ray absorptiometry (DXA) for the measurement of bone mineral density (BMD) at baseline (T0) and after 1 year of treatment with TCZ (T2). Serum levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IL-6, serum CrossLaps (CTX-I), osteoprotegerin (OPG), receptor activator of nuclear factor κβ ligand (RANK-L), and component of the Wnt/β-catenin signaling pathway dickkopf-1 (DKK-1) were measured at baseline (T0), after 6 months (T1) and after 1 year (T2) of treatment.
Exclusion criteria were as follows: any previous disease able to impair bone metabolism and concomitant or previous treatments with bisphosphonates, teriparatide or denosumab.
Bone mineral density measurement
Bone mineral density (BMD) was measured at lumbar spine (L1–L4) and whole left femur using DXA (Lunar Expert version 1.72); BMD was expressed in g/cm2. Every day an experienced physician performed quality control of the device. Diagnosis of osteoporosis was established when T-score deviated below –2.5 standard deviations compared with reference population according to the World Health Organization (WHO) criteria. The same densitometer was used for the T0 and T2 BMD evaluations.
Laboratory assessments
Erythrocyte sedimentation rate and CRP were collected at baseline (T0) and after 1 year (T2). Bone turnover markers were evaluated at baseline (T0), after 6 months (T1) and after 1 year (T2).
The receptor activator of nuclear factor κβ ligand, DKK-1, OPG, CTX-I and IL-6 were assessed by standard ELISA immunoassay in accordance with the manufacturer’s recommendations.
Statistical analysis
The software packages SPSS (ver. 16.0), GraphPad (ver. 9.4.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com) and MedCalc (ver. 9.0.1) were used for the analysis. Variables distributions were checked with the Shapiro-Wilk test. Differences of mean values were evaluated with the Mann-Whitney U test and Wilcoxon’s test. The Kruskal-Wallis test was used to compare means of more than two groups, then post hoc Dunn’s analysis was performed. Spearman’s rank test was used to identify correlations between variables. A p-value < 0.05 was considered significant.
Bioethical standards
This study was conducted in accordance with the Declaration of Helsinki and its late amendments, moreover it was approved by the Local Ethical Committee (Rhelabus, protocol number 22271).
Patients
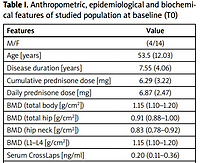
We enrolled 18 patients: 14 were female, 4 male, mean age 53.5 ±12.03 years, mean disease duration 7.55 ±4.06 years; 6 of them interrupted TCZ treatment due to loss of efficacy.
Data of 12 patients were used in the statistical analysis. In the sample group, one patient had osteoporosis, nine osteopenia and two normal BMD. All patients had previously failed at least one anti-TNFα antagonist. At baseline, two patients were in therapy with methotrexate and one with leflunomide. Patients’ epidemiological features are summarized in Table I.
Table I
Anthropometric, epidemiological and biochemical features of studied population at baseline (T0)
Results
Changes of bone mineral density measurement at T0–T2
After 1 year of TCZ a significant increase of lumbar spine (L1–L4) BMD was documented (p = 0.02). Also, albeit not in a significant manner, an increase of total hip BMD as well as total body BMD was recorded (Table II).
Changes of biochemical markers at T0, T1 and T2
No significant difference in IL-6, RANK-L, DKK-1, OPG and serum CrossLaps levels at T0, T1 and T2 were found. Nevertheless, a decreasing trend was evidenced in serum CrossLaps, dickkopf-1 (DKK-1) and IL-6 levels. The small sample size did not allow these changes to be defined as significant. Interleukin 6 displayed a reduction of 4.25 pg/ml (22.4%), whereas DKK-1 showed a reduction of 5.91 pmol/l (33.3%) (Table III). At T2 significant reductions of ESR (33.05 ±28.05 vs. 17.09 ±27.05 mm/h, p < 0.02) and CRP (5.17 ±10.49 vs. 0.4 ±0.78, p < 0.01) were noted compared to baseline.
Table III
Markers sera modifications compared to baseline (T0), 6 months (T1) and end of study (T2)
Bone mineral density and bone turnover markers
A negative correlation between lumbar spine BMD and IL-6 (–0.70, p = 0.04), as well as spine BMD and serum CrossLaps (r = –0.59, p = 0.05) were identified at 6 months from baseline. A positive albeit not significant correlation between total hip and femoral neck BMD and OPG (r = 0.45) was documented. No significant correlation was found between bone mineral density and bone markers after 1 year of therapy with TCZ (Table IV).
Table IV
Spearman correlation coefficient between BMD and markers after 6 (T1) and 12 months (T2)
Correlation between cumulative and daily dosage of prednisone and bone mineral density and serum markers at T2
A positive correlation between cumulative dose of prednisone and BMD at femoral neck (r = 0.67, p < 0.05) and total hip (r = 0.40, p = 0.33) was assessed. An inverse correlation between total hip BMD (r = –0.46, p = 0.35) and lumbar spine BMD (r = –0.74, p < 0.05) was related to the daily dose of prednisone intake (Table V).
Table V
Spearman correlations between cumulative/daily dose of prednisone, bone mineral density and sera markers after 1 year of therapy (T2)
Discussion
Rheumatoid arthritis patients have a greater prevalence of osteoporosis and bone loss compared to healthy individuals of same age and gender [1]. Age, alongside with low physical activity and low estrogens levels, is a well-known cause of secondary osteoporosis in healthy subjects; the same pathological mechanisms act in RA patients, enforced by the high inflammatory load as well by the chronic GC therapy, impairing bone metabolism.
Several studies on the immunological mechanisms have provided new insights in understanding inflammatory and immunologically mediated bone loss. It has been demonstrated that high levels of rheumatoid factor and anti-citrullinated antibodies (ACPA) are linked to an accelerated juxta-articular as well systemic bone loss [4, 5] by directly promoting pre-osteoclast differentiation via RANK-L, thus leading in an uncoupling between bone resorption and formation.
Moreover, a high inflammatory load is capable of unbalancing bone homeostasis acting on the RANK-L/OPG ratio and affecting simultaneously bone formation and resorption [6, 7]: osteoclasts differentiation is enhanced by tumor necrosis factor α (TNF-α) through a mechanism involving activation of RANK-L [8], while osteoblastogenesis is contemporary reduced by DKK-1 induced by TNF-α [9].
The decrease of Wnt pathway activation leads to reduced differentiation and function of osteoblasts and to reduced production of OPG, causing an imbalance in the RANK-L/OPG ratio. Moreover, IL-1, IL-6 and IL-17 are capable of directly increasing RANK-L expression, thus promoting osteoclastogenesis and bone resorption [8, 10, 11].
The IL-6, whose serum levels have a significant inverse correlation with T-score and BMD [8], is a potent stimulator of osteoclast-induced bone resorption playing a synergic role with TNF-α [12] and IL-1 [10].
Indeed, IL-6 is capable of influencing osteocyte metabolism: even in physiological conditions, apoptotic osteocytes secrete IL-6, which promotes expression of the adhesion molecule ICAM-1 on osteoclast precursors. Moreover, this cytokine increases the differentiation of Th17 cells [13] which in turn enhance osteoclastogenesis and osteoclast activity.
Furthermore, several papers have documented the deleterious effects of IL-6 on bone: thus, IL-6 inhibition may be a promising tool in controlling systemic inflammation and bone loss in RA. The first study on IL-6 effects on bone metabolism was performed in vitro by Axmann et al. [14] demonstrating that IL-6R neutralization was able to reduce osteoclast differentiation and bone loss in murine cell cultures stimulated with RANK-L.
Subsequently, different studies performed in humans evaluated the effectiveness of TCZ, the first registered monoclonal antibody anti-IL-6 receptor, in reducing osteoclasts activation. In 2010, a sub-study of OPTION trial [15] detected a significant increase of bone formation markers and a reduction of bone resorption markers in patients treated with TCZ.
In 2012, the RADIATE study [16] confirmed these findings: TCZ significantly reduced the levels of CTX and simultaneously augmented neoformation markers. Another study investigating the effect of TCZ on bone metabolism, performed by Terpos et al. [17], reported the reduced serum levels of DKK-1 and increased OPG/RANK-L ratio after 2 months of TCZ.
Furthermore, a histological evaluation in 10 RA patients documented an increase in OPG bone marrow expression after TCZ treatment [18]. Subsequent BMD evaluations of RA patients treated with TCZ were made by Kume et al. [19] in 2014 and Briot et al. [20] in 2015, although these researchers reported different outcomes: the former demonstrated an increase in BMD of osteopenic patients, while the latter did not find any significant change. Indeed, other evidence suggests a synergistic effect between denosumab and TCZ, which appeared more effective than TNF-α or abatacept plus denosumab in preventing bone loss in RA patients [21].
A more recent prospective study published by Chen et al. [22] revealed a positive effect of bone metabolism through the IL-6 inhibition in RA patients: after the TCZ treatment an increase of femoral BMD as well as a CTX reduction was documented, but the osteocalcin and the P1NP levels did not change from the baseline. Interestingly, the abovementioned findings were observed only in ACPA positive patients, underlining the close correlation between ACPA and bone loss in RA.
Moreover, other than being associated with a high fracture risk and bone metabolism impairment, RA is characterized by an increased cardiovascular risk [23]. Some papers suggested that high levels of IL-6 in RA may lead to a QTc prolongation [24] thus leading to an increased risk of developing malignant arrhythmias such as torsades de pointes by acting on h-ERG potassium channels [25].
Several studies suggest that osteoporosis and cardiovascular diseases are driven by the same pathogenetic pathways [26] and it would be interesting in future to assess in real life scenarios if blocking IL-6 activity both in bone and cardiac muscle would produce a concomitant reduction in cardiovascular events and fractures in RA patients.
In our cohort, we observed a significant raise of lumbar spine BMD and the raise of total hip BMD, without further loss of femoral neck and total body BMD, confirming the bone sparing effect of IL-6 antagonism.
These results may be explained by the reduction of cytokine inflammatory burden, the reduction of bone resorption via RANK-L system and the increase of bone formation markers due to the decreased activity of DKK-1. In other words, the beneficial effects of TCZ therapy may re-equilibrate the RANK-L/OPG ratio and re-establish a normal bone turnover, thus reducing bone loss.
We obtained similar results in an our previously published paper [27]; rituximab (RTX), after an observational period of 18-months, was associated with an increase of lumbar spine BMD in RA patients. Moreover, the results reported in this paper may be explained by the concomitant reduction of ESR and CRP at end of study compared to baseline, strengthening the hypothesis that the low disease activity or the remission may exert bone-sparing effects in RA patients. Nevertheless, TCZ is a molecule which intrinsically suppresses IL-6 and CRP production, therefore disease activity in this subset of patients should be evaluated with other tools.
Indeed, we found a negative correlation between daily dose of prednisone and lumbar spine BMD and a positive correlation between cumulative dose of prednisone and femoral BMD: this evidence could be explained by the fact that low doses of prednisone for a long time may produce beneficial effects on bone metabolisms by inhibiting the inflammatory cascade.
An improvement of BMD in RA patients undergoing a GC treatment was already reported by Angeli et al. [28] and Wijbrandts et al. [29]: the former documented an improvement of hands BMD in early RA patients treated with GCs, while the latter observed an increase of femoral BMD in patients undergoing GC therapy.
Furthermore, these findings are in accordance with those previously reported by our research group: intravenously administered GCs does not negatively affect BMD of RA patients [30].
Study limitations
The present study has several limitations: the small sample size, the lack of a control group and the lack of disease activity measurements do not allow the generalization of our results.
Conclusions
We suggest that TCZ may exert osteoprotective effects on BMD in RA individuals and we believe that these effects are grounded in the reduction of disease activity and in RANK-L/OPG ratio normalization, thus reducing inflammatory burden.
Indeed, the reduction of ESR and CRP at T2 compared to baseline may reflect an inverse relationship existing between disease activity and BMD; therefore, larger prospective studies are needed to confirm these findings.