Introduction
Spondyloarthropathies (SpA) are a group of chronic inflammatory rheumatic diseases with specific clinical symptoms. The four groups of SpA in adults are ankylosing spondylitis (AS), reactive arthritis (ReA), enteropathic arthritis (inflammatory bowel disease), and psoriatic arthritis (PsA). Peripheral arthropathies associated with inflammatory bowel disease or psoriasis are not always associated with spondylitis and can be considered as a separate disease [1, 2].
Another disease in this group is juvenile spondyloarthropathy, which is similar to ankylosing spondylitis (AS) and usually persists until adulthood. The major forms of SpA include sacroiliac inflammation, inflammation in the spinal bones (spondylitis), inflammation of the tendon junction (enthesitis), and inflammation of the anterior chamber of the eye (uveitis). There are other manifestations within each type of SpA. As mentioned, SpA have a remarkable clinical overlap with each other and are considered as a group of relevant diseases [3, 4].
Magnetic resonance imaging (MRI) studies indicated that bone marrow edema, arthritis, and joint fibrosis are largely resolved in the sacroiliac joint, vertebrae, and peripheral joints. Similar results have been published in randomized controlled trials of five drugs and many open-label trials. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) value decreased in more than 50% of patients. The therapeutic response continued over time, and partial or complete improvements were not uncommon.
Due to the cost of treatment and potential and serious side effects and long-term adverse effects of these drugs, their use was limited to patients with a definite diagnosis and active disease. Active disease (BASDAI more than 4 out of 10 and expert opinion) did not indicate a satisfactory response to treatment with at least two types of nonsteroidal anti-inflammatory drugs (NSAIDs) [5].
In this randomized controlled trial study, we evaluated the effect of intra-sacroiliac joint methylprednisolone injection on the recovery of patients with SpA who were reffered to the Rheumatology Clinic of Golestan Hospital, Ahvaz in 2020.
Material and methods
Study design
The patients were randomly allocated to one of two groups (treatment and placebo group) and the contents of the injection were hidden from both the patient and the physician who administered the injection. One researcher prepared the injection and presented it to the operation room in a prefilled, numbered syringe.
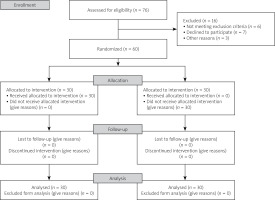
After all data collection was done, the treatment key was unblinded. In this randomized control trial study 60 patients with SpA (30 patients in the intervention group and 30 patients in the control group) were referred to the Rheumatology Clinic of Golestan Hospital, Ahvaz from January 2020 to December 2020. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented in Figure 1.
The patients were selected by a rheumatologist based on ACR criteria. All individuals were subjected to clinical evaluation and physical examination, and their history, demographic characteristics, and medical history were collected and recorded in a data collection checklist.
The diagnosis of spondyloarthropathy was confirmed according to the criteria of the European Spondyloarthropathy Study Group (ESSG) and the presence of pelvic pain by a rheumatologist. Diagnosis of SpA was based on HLA-B27 positivity and MRI of sacroiliac joints (SIJ) as well as the Schober test and fingertip-to-floor (FTF) test.
Data collection
Data collection included age, gender, weight, BMI, occupation, duration of the disease (year), underlying disease, medications, type of inflammatory disease (AS, PsA etc.), disease severity, and grade of sacroiliitis (grade 0 to 4 based on graph) and pain intensity (using Visual Analogue Scale – VAS) before the intervention. Laboratory findings including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were also collected and recorded.
Patients over 18 years old with the presence of inflammatory pain in the SIJ line due to SpA for at least 3 months (nocturnal pain and stiffness in the morning) despite treatment with NSAIDs, disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (glucocorticosteroid – GC) or sulfasalazine (in cases of peripheral joint involvement) were selected.
Patients with spinal infections (e.g. brucellosis), infectious arthritis, local infection at the injection site, receiving anticoagulant therapy, with uncontrolled diabetes due to GC side effects, the presence of avascular necrosis in the femoral head, the presence of any skin damage or defect at the injection site, any previous injections in the last three months, any additional therapeutic interventions during the study period that affect the treatment (injections or surgery), hip dysfunction, or symptoms of lumbar radiculopathy were excluded from this study.
Intervention
Eligible patients were selected based on inclusion criteria and were divided into two groups by matching age, gender, and disease severity. In the intervention group, in addition to treatment with NSAIDs (inflammatory dose) and sulfasalazine (2 to 3 g/day), methylprednisolone was injected at the beginning. For all patients in the intervention group, 40 mg of methylprednisolone was injected into each SIJ.
All patients in the control group were treated with NSAIDs (inflammatory dose) and sulfasalazine (2 to 3 g/day). Patients in the control group did not receive any methylprednisolone injections and they received routine therapeutic management. Standard medications were prescribed based on Kelley’s 2020 reference [6]. In case of no response to treatment in any of the patients in both groups, anti-TNF therapy was prescribed and patients were not deprived of other treatments.
Methylprednisolone injection
The patient was placed in the prone position to inject the drug. After skin disinfection, the ultrasonographic examination was performed with a linear probe at 6 to 12 MHz. Focus and penetration depth was adjusted based on anatomical landmarks and return on investment (ROI).
After the SIJ cleft was identified, a 22 G spinal needle was inserted mediolaterally at a 45-degree angle, about 2 to 3 cm below the posterior superior iliac spine (PSIS), under ultrasonography with a freehand technique, and passed through the sacrum and ilium until ligamentous resistance was felt and GC was injected into the joint space. After the injection, the patient’s symptoms and pain intensity were evaluated to detect any adverse reactions to the injection.
Evaluating the effectiveness of treatment
The patients’ pain intensity and symptoms were assessed in the 2nd, 4th, 6th, and 8th weeks after GC injection. Patients were evaluated by another rheumatologist who did not know the grouping of patients. Patients’ pain intensity and symptoms were assessed using the VAS.
To assess the severity of the pain at intervals, telephone calls were made to patients and they were asked about the severity of pain, symptoms, and complications. The VAS is a self-report method for assessing the severity of pain so that the patient gives a score between zero (no pain) to 10 (maximum pain).
Other assessments included finger-to-floor and Schober tests, morning stiffness, and the BASDAI scores (the disease activity questionnaire contains 6 questions regarding subjective symptoms during the week before answering the questions and each question is scored on a scale of 0 to 10 where 0 means none and 10 indicate very severe). Injection complications such as injection site infection were also evaluated. Finally, the effectiveness of treatment was compared between these two groups using related tests.
Statistical methods of data analysis
Sampling was done randomly and the sample size was determined based on a 95% confidence interval and 80% power and according to similar studies in each group included to 30 cases [7–9]. The normality test indicated the distribution was normal so the Wilcoxon signed-rank Z test was not used because the data follow a normal distribution and Student’s t-test and ANOVA were used, SPSS software version 22 was used for statistical analysis. The obtained data were analyzed by descriptive statistics including mean, standard deviation, frequency, and frequency percentage. The normality of the data was checked by the Kolmogorov-Smirnov test.
Analysis of variance with repeated measures was used to evaluate the effectiveness of treatment and the Wilcoxon signed-rank Z test was used to compare the results before and after injection. The χ2 test (or Fisher’s exact) and Pearson’s correlation were used to examine the relationship between variables. The significance level in the tests was considered 0.05.
Ethical standards
All procedures performed in studies involving human participants complied with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethic code: IRAJUMS.HGOLESTAN.REC.1399.063. All patients signed informed consent to participation in this study.
Results
The mean and standard deviation of the age of the subjects in the control and case groups were 27.86 and 32.13 years, respectively. Eight patients were female and 22 were male in the control group and 1 female and 29 male in the case group. Using the one-way analysis of variance test, the difference between the VAS variable in the case and control groups was compared at the initiation, 2 weeks, 4 weeks, 6 weeks, and 8 weeks later. There was a significant association in VAS (p-value < 0.0001), as shown in Table I.
Table I
Difference between the VAS variable in the case and control groups
| Groups | VAS | Initiation | 2 weeks | 4 weeks | 6 weeks | 8 weeks | p-value |
|---|---|---|---|---|---|---|---|
| Intervention | Mean ±SD | 9.7 ±0.59 | 3.3 ±1.46 | 3.54 ±1.73 | 3.2 ±1.4 | 3.28 ±1.6 | < 0.0001 |
| Control | Mean ±SD | 8.53 ±1.04 | 8.23 ±1.16 | 8.23 ±1.16 | 8.3 ±1.26 | 8.23 ±1.43 |
There was a significant difference (p-value < 0.0001) between the BASDAI variable in control and case groups, in the beginning, 2 weeks, 4 weeks, 6 weeks, and 8 weeks later, as shown in Table II.
Table II
Correlation between BASDAI variable in case and control groups
| Groups | BASDAI | Initiation | 2 weeks | 4 weeks | 6 weeks | 8 weeks | p-value |
|---|---|---|---|---|---|---|---|
| Intervention | Mean ±SD | 6.83 ±1.26 | 1.1±2.52 | 1.3 ±2.66 | 1.1 ±2.52 | 1.26 ±2.58 | < 0.0001 |
| Control | Mean ±SD | 7.26 ±1.04 | 7.13 ±1.13 | 6.96 ±1.18 | 7.06 ±1.25 | 7.03 ±1.32 |
There was a significant difference between intervention and control groups in FTF variables at the initiation, 2 weeks, 4 weeks, 6 weeks and 8 weeks later (p-value < 0.0001), as shown in Table III.
Table III
Coefficient between fingertip-to-floor variable in case and control groups
| Groups | FTF | Initiation | 2 weeks | 4 weeks | 6 weeks | 8 weeks | p-value |
|---|---|---|---|---|---|---|---|
| Intervention | Mean ±SD | 18.43 ±2.77 | 3.06 ±7.16 | 3.3 ±6.84 | 2.56 ±5.94 | 2.9 ±6.07 | < 0.0001 |
| Control | Mean ±SD | 15.86 ±4.6 | 15.63 ±4.64 | 15 ±4.92 | 15.4 ±4.95 | 15.33 ±5.01 |
The relationship between the Schober variable was studied by χ2 test, and it was significant (p-value < 0.0001), as shown in Table IV.
Table IV
The relationship between Schober variable in intervention and control groups
[i] Table IV indicates χ2 test for Schober test variable.
The relationship between Patrick’s test in these groups was significant based on the χ2 test (p-value < 0.0001), as shown in Table V.
Table V
Correlation between Patrick’s test in intervention and control groups
[i] Table IV indicates χ2 test for Patrick’s test.
Discussion
A recent study was conducted in two similar groups in terms of age, gender, and numbers, the degree of pain sensation according to the VAS pain scoring criteria at the time of admission, and also during 8 weeks. The control group, which received the usual treatment for spondyloarthritis, and the intervention group, which received methylprednisolone (GC) injection under the sonography guide, had similar VAS pain scores at baseline. The results of the study showed that in the control group there was no significant difference in the pain score compared to the admission.
However, in the intervention group, a significant reduction in pain was reported by patients according to VAS criteria and the results were significantly different after two weeks [10]. Sadreddini et al. [11] studied 29 SpA patients. In this study, they injected triamcinolone acetonide (GC) 40 mg into each SIJ of the patients and followed-up the patients 4 weeks later.
The study indicated that SIJ injection was effective, and a significant improvement was reported. Another study conducted by Maugars et al. [12] evaluated the efficacy of a sacroiliac GC injection in SpA. This controlled double-blind study was performed on 10 patients (13 joints) with painful sacroiliitis. Patients were divided into two groups of GCs injections (6 joints) and placebo (7 joints).
The results showed that in the first month after injection, 5 of the 6 SIJ had a reduction of more than 70% in pain assessment but in the placebo group, no reduction in pain was reported and the difference between these two groups was significant (p < 0.05). In 86% of cases the drug was well tolerated and no side effects were reported. As a result, this technique is considered safe and highly effective and can be widely used for patients with NSAIDs contraindications or complications [12].
In 2019, Farhoud et al. [13] conducted a study in Egypt to evaluate the therapeutic benefits of intra-sacroiliac injection. This study was performed on 20 adults with sacroiliac pain for various reasons and candidates for SIJ GCs injection and local anesthesia. Patients received an injection of triamcinolone acetonide (40 mg/ml) and 4 ml mepivacaine under a computed tomography (CT) guide at each SIJ and were followed for 6 months. Patients’ pain intensity was assessed using the VAS scale.
The results showed that after 6 months of follow-up, inflammatory back pain was significantly reduced in 85% of patients (7.50 vs. 2.00). Consequently, GC injection under CT or fluoroscopic guidelines, with minimal invasion, was reported as an effective treatment with good results and can be used for patients with SIJ at least in the short term [13].
In this study, both intervention and control group had similar VAS pain scores at the baseline. However, there was a significant difference in the amount of pain after two weeks, and a significant reduction in pain was reported by the intervention group based on the VAS.
Pain intensity was also assessed based on BASDAI and the results of the study showed that patients had similar scores at the time of admission but two weeks later the case group, reported a significant decrease in pain expression, which remained the same until the end of 8 weeks. The finger-to-floor test also showed a significant decrease in the intervention group after 2 weeks.
The sacroiliac joint block is a local anesthetic injection (IA) into the SIJ, which can be used as a diagnostic test to confirm the presence of SIJ-related pain. The results of this study disagree with Hartung et al. [14]. They used ultrasound-guided injections of 40 mg of triamcinolone and 0.78 mg of gadolinium in 20 SIJ of 14 consecutive patients who were reported as active sacroiliitis patients. The authors reported that IA SIJ injections seem to be technically challenging despite using ultrasound. However, peri-articular deposition of triamcinolone was effective in control-ling the pain and symptom [14].
The guide CT method is currently used to inject SIJ IA in routine clinical applications. Although fluoroscopic and CT-guided techniques have the advantages of being safe and effective, the harms of radiation exposure limit their use [15–17].
Slipman et al. [18] conducted a study using ultrasound imaging which has been used as an alternative method of inserting a needle into the SIJ space recently. Color Doppler ultrasound was used to increase the success rate and confirm IA during the epidural injection in this study. Color Doppler imaging has been reported to be suitable for precise control of needle tip placement in the tail epidural injections [18].
Jee et al. [19] conducted a study on 55 patients using an ultrasound guide; 54 patients showed positive spectral change through contrast injections and 48 of them received IA space injections which were successfully confirmed by fluoroscopy. Contrast color was observed outside the capsule in the remaining 6 individuals due to needle tip movement during injection procedures and the results of this study were similar to the recent study. This study was parallel with our study too. This work confirmed the benefit of ultrasound guidance in SIJ injection [19].
Sacroiliac joints guided ultrasound injections are routinely administered in some clinical institutions but have not been widely used worldwide because they are considered as a novel emerging technology. The main reason for the limited use of this technique is that is used as an experimental method and is ineligible for subsequent financial repayments by insurers (in most countries) [20, 21].
Intra-sacroiliac joint injection with CT and fluoroscopy is accurate, but it is time-consuming and expensive, and radiation exposure limits its use. Fluoroscopy has been the preferred imaging technique for SIJ injection. Using such technique, this method is quite accurate, because several performed experi-ments reported it to be 98% and 97% successful, respectively.
However, Hendrix et al. [22] reported that radiation exposure was 12–30 mGy/min for the skin and 0.6–1.6 mGy/min for the gonads by fluoroscopy. The results of this study confirms the efficacy of using ultrasound in SIJ guidance and this fact is parallel with our study [21].
Murakami et al. [23] conducted a study on 50 patients and evaluated the impact of intraarticular lidocaine injections for sacroiliac joint pain. A significant difference was observed between the groups of ultrasound and fluoroscopy for the accuracy of IA injection, without any difference in the effectiveness of the treatment. This finding is similar to our findings in this study [23].
Luukkainen et al. [24] conducted a study on 24 patients to evaluate the impact of periarticular GC SIJ injection in non-spondyloarthropathic patients and they concluded the periarticular injection of methylprednisolone can be useful in decreasing the pain in the SIJ. This study supports the results of our study and its findings are similar to our report [24].
However, the efficacy sometimes shows different values in different studies, which may be due to the diverse nature of the source of the pain. Many studies have shown that the neural pathways of nerve fibers and pain receptors are located not only in the joint capsule but also in the posterior ligament tissue, so they will serve as additional sources of sacroiliac pain.
Due to limited information on medical history, physical examination, and imaging techniques, a diagnostic block with fluoroscopic or CT guidance is the only method for definitive diagnosis or rejection of SIJ as a source of pain [25, 26].
Several studies have evaluated the accuracy of SIJ-guided ultrasound injections. The accuracy in these studies varies from 40% to 100%. This wide range can have many explanations including injection experience and injection method or the technique is not standard in all studies and different approaches have been published [7, 14, 19].
In 2003, Pekkafali et al. [11] reported an approximately 77% success rates for intra-articular injections using fluoroscopically confirmed ultrasound guides. The results of this study are similiar to our findings [7].
Bollow et al. [27] conducted a study in Germany to evaluate the effectiveness of GC injections in the sacroiliac joint under the guidance of CT in patients with spondyloarthropathy. Patients were followed up by an MRI evaluation.
In this study, 103 intra-sacroiliac joint GC injections (40 mg of crystalline per joint) were performed under CT guidance for 66 patients. Not all patients responded to NSAIDs therapy for 4 weeks. All patients underwent follow-up for 10 to 12 weeks. Pain intensity and complications before and after injection were assessed from 0 to 10 using the analog pain scale. Magnetic resonance imaging was performed with improved contrast for all patients before the intervention and in 38 patients for an average of 8 months after injection.
The results showed that in the second week after injection, 92.5% of patients had a significant reduction in pain intensity (8.8 vs. 3.3) and this improvement continued until the tenth month. Magnetic resonance imaging results also showed a significant reduction in inflammation. As a result, SIJ injection of GCs under CT guidance can be an effective treatment to reduce pain in SpA patients. This study is similar to our study and the conclusion is parallel to our report [27].
Study limitations
This was a preliminary study, and our patient population was small but was able to show that ultrasound guidance could be used accurately to complete the SIJ injection. Currently, further observation of patients is continued and the expansion of the research group is planned.
We noticed that the individual’s skill is important and increases the difficulty in directing the shooting the imaging techniques, and this is reflected in the results. However, there is an expert training program for physicians who want to follow this procedure, and additional training is required.
Conclusions
In this study, the SIJ injection method of methylprednisolone with ultrasound guidance showed a significant improvement in pain relief and function, and patient satisfaction scores compared to the control group. Therefore, the SIJ injection method with an ultrasound guidance may be a reliable imaging technique for SIJ therapeutic injection.
Advantages such as comfort and risk-free radiation make ultrasound preferable to other methods to control procedure of injection.