Introduction
Rheumatoid arthritis (RA) is a persistent autoimmune disease characterized by chronic inflammation and polyarthritis of unknown origin, impacting multiple systems in the body [1]. According to field studies, the prevalence of RA is estimated to be around 70% of the population [2]. This condition has deleterious effects on the synovium, progressing from inflammatory variations to the destruction of bone and cartilage, potentially leading to systemic inflammation affecting various organ systems in the human body [3]. Rheumatoid arthritis considerably diminishes patients’ quality of life, hinders their employment opportunities, and imposes a substantial economic burden [4]. Vitamin D deficiency has been linked to the development of autoimmune diseases and multiple sclerosis [5]. Thus, numerous studies have indicated that vitamin D, in addition to its role in regulating phosphorus and calcium metabolism, plays a crucial part in modulating immune and anti-inflammatory activities [6]. Epidemio-logical data consider vitamin D deficiency as a risk factor for the progression of chronic diseases and autoimmune conditions [7]. Conversely, vitamin D deficiency plays a role in the pathogenesis and increased severity of RA [8]. The inflammatory consequences of RA contribute to pain and raise disease activity, revealing a negative correlation between vitamin D levels and the extent of fatigue, disease activity, and autoantibody production [9]. Fatigue is considered one of the most incapacitating symptoms by patients with RA, which is why assessing fatigue levels provides important insights to understand the disease outcomes [10]. The cause of fatigue in RA remains poorly understood since many patients continue to experience fatigue even after achieving remission or reduced disease activity [11]. Fatigue in patients with RA, as well as in other clinical cases, is associated with pain, anxiety, depression, sleep disorders, and obesity [12]. A cross-sectional study conducted in Tripoli, Libya aimed to investigate vitamin D intake, status levels, and associated factors in the Libyan population. The findings revealed that the prevalence of vitamin D deficiency was 55.63% among the participants. In other words, the percentage of vitamin D deficiency was 25.58% in males and 79.26% in females [13]. The objective of the current study was to investigate the effect of vitamin D supplementation therapy in managing disease activity and fatigue in RA patients.
Material and methods
Study population
The study exclusively included newly diagnosed RA patients with less than one year of disease and age over 18 years. The population was collected from the outpatient departments of medicine and rheumatology in Tripoli Central Hospital, Libya, between May 2018 and December 2019. Rheumatoid arthritis patients fulfilled the classification criteria based on the 2010 classification criteria for RA established by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) [14]. In addition, the patients were chosen to participate and their family members were recruited to supervise drug consumption after obtaining their agreement.
Excluded patients received glucocorticosteroids (GCs) during the last year, patients with diseases similar to RA features such as psoriatic arthritis, acute viral polyarthritis, polyarticular gout, or calcium pyrophosphate deposition disease, hyperthyroidism, liver diseases, Cushing’s disease, diabetes mellitus, kidney diseases, trauma, those with a history of previous intestinal surgery (to avoid interference with absorption of drugs) and allergy to disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX), hydroxychloroquine (HCQ), and sulfasalazine (SF) as well as vitamin D. Also women during pregnancy and breastfeeding were excluded. These excluded criteria were implemented to ensure that the study participants met specific eligibility criteria and to minimize confounding factors that could affect the study outcomes.
Study design
A prospective randomized clinical trial was conducted at clinics of rheumatology in Tripoli Central Hospital, Libya. All eligible patients were on DMARD treatment and randomized according to a 2.4 : 1 ratio to receive 50,000 IU of vitamin D (cholecalciferol) once a week or received a DMARD without vitamin D supplementation. Methotrexate was given as a single dose once a week in an initial minimal dose of 10 mg per week, with 200 mg of HCQ used daily, and 1 g of SF given twice a day. Internationally, MTX at low doses remains the agreed first-line DMARD for the therapy of RA without toxicity correlation [15, 16]. Both study groups were administered drugs and monitored for 12 weeks.
Methods
At baseline, the rheumatologist investigator observed the patients, collected demographic data (age and sex), and recorded their medical history. Moreover, clinical examinations including tender joint count and swollen joint count were performed. Some blood tests were conducted including assessments of blood counts, renal and liver function, serum vitamin D and calcium levels, and evaluation of disease activity markers such as erythrocyte sedimentation rate (ESR).
To determine the effectiveness of vitamin D therapy, the study considered various parameters, including: total number of tender joints, total number of swollen joints, assessing Disease Activity Score with 28-joint count (DAS28), and fatigue levels were calculated. The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score is used to assess fatigue according to a global measurement range from 0 to 52, with higher scores representing less fatigue. A normative score for the FACIT-F is ≥ 40.1 [17].
Laboratory investigations
C-reactive protein (CRP), calcium (Ca), and ESR were assessed. The laboratory investigations were conducted on the samples using specific methods: rheumatic factor (RF) and CRP were analyzed with the Cobas 6000 c501 module by turbidimetry from Roche Diagnostic, Germany. Additionally, the ESR (in 1 h) was determined using the Wintergreen method. The ARCHITECT c 8000 was used in analyzing the sample of vitamin D level, and the test that was used for measurement is quantitative chemiluminescent microparticle. Then, the ARCHITECT i System was used for calibrating the vitamin D assay (25-OH vitamin D) in human serum and plasma.
Sample size calculation
The sample size was calculated based on an expected average of 10%. The differences in disease regression (serum vitamin D, DAS28 and FACIT-F were measured by a pooled standard deviation (SD) of 15% between the two study groups [19]. The sample size calculation was done at a 95% confidence interval (95% CI) and with 80% power. The process of calculation was performed based on expected outcomes; thus, the sample size was 68 RA patients.
Statistical analysis
IBM SPSS version 26.0 was used for statistical analysis. Data are displayed as mean ±SD, or number and (percentage). The normal distribution of variables was examined through analytical methods (Kolmogorov-Smirnov-Shapiro test). To compare independent groups, the independent t-test was conducted. Correlation analyses employed and depended on the Pearson test. The χ2 test was used for categorical variables. The predetermined threshold for statistical significance was set at p < 0.05.
Bioethical standards
The research methodology and study design adhered to ethical guidelines, aligning with the Declaration of Helsinki [17], and obtained approval from the Institutional Review Board (IRB) under reference number BEC-BTRC 5-2020 at Tripoli Central Hospital, Libya.
Results
A total of 68 RA patients were chosen to complete the study. Forty-eight of them were given vitamin D3, i.e. 50,000 IU of cholecalciferol (group A), and 20 received DMARD drugs alone (group B). The age of patients ranged from 19 to 70 years. The majority of patients were female 75% and 25% were male. The average duration of illness was 4.23 ±2.35 months in group A and 4.45 ±2.5 months in group B. There was no statistically significant difference between the two groups with respect to age, sex or duration of illness (p > 0.05). At baseline, the means of serum ESR, CRP, Ca and vitamin D levels were 41.25 ±26.8 mm/h, 11.50 ±8.31 mg/l, 8.9 ±1.3 mmol/l and 7.93 ±3.84 ng/ml respectively in group A and 34.9 ±15.12 mm/h, 8.96 ±6.73 mg/l, 9.13 ±0.27 mmol/l and 7.51 ±4.06 ng/ml respectively in group B. The means DAS28 score and FACIT-F score were 5.3 ±1.22 and 25.22 ±5.48 respectively in group A and 4.83 ±8.8 and 24.58 ±4.12 respectively in group B. Statistically, there was no significant difference between the two study groups in terms of any of these parameters (p > 0.05; Table I).
Table I
Baseline demographic, clinical and biochemical profile of rheumatic arthritis patients in two study groups
At 12 weeks, patients in group A had a significantly higher mean vitamin D level (nmol/l) and FACIT-F score as compared to those in group B, with a p-value < 0.05. Mean vitamin D level was higher (24.21 ±4.81 nmol/l) in group A, compared to 5.76 ±3.36 nmol/l in group B. Conversely, the mean FACIT-F score was only slightly less than the “normal” value of fatigue (FACIT-F = 39.36 ±6.15) in group A, compared to the “abnormal” positive value of fatigue (FACIT-F < 27.75 ±4.41) in group B (Table II).
Table II
Comparison of overall change parameters in two study groups at final evaluation in two study groups
There was no statistically significant difference between group A and group B regarding the parameters ESR, Ca, CRP levels and DAS28 score, with p > 0.05. But it was clear that there was a decrease in ESR, CRP levels, and DAS28 score and a slight increase in Ca level parameters in the two study groups at the end of the evaluation (Table II).
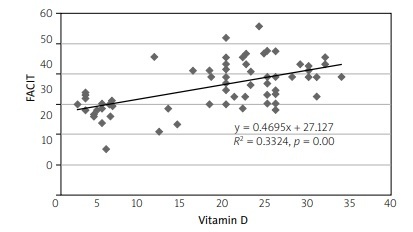
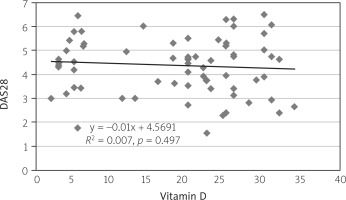
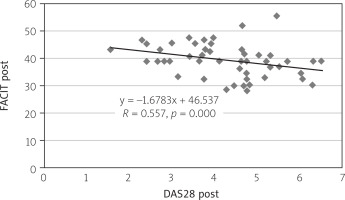
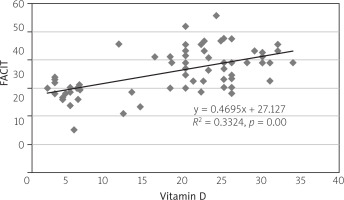
Concerning correlation, there was a positive correlation between the FACIT-F and vitamin D levels with the value of correlation coefficient R = 0.577, p = 0.001 (Fig. 1). Moreover, there was a weak inverse correlation between the DAS28 score and vitamin D level with R = –0.024, p > 0.05 (Fig. 2). Finally, the correlation test identified a significant inverse relationship between DAS28 and FACIT-F after intervention in the vitamin D-supplemented group (R = 0.557, p = 0.000; Fig. 3).
Fig. 1
Scatter diagram showing positive correlation between the FACIT-F score and vitamin D levels with value of the correlation coefficient R = 0.577, p = 0.001, after the patients received the supplement vitamin D.
Discussion
Numerous studies have indicated that there is a relationship between the level of vitamin D and the activity of the immune system. In addition, these studies have shown a strong association between high consumption of vitamin D and a reduced risk of developing RA [8, 20]. Consequently, in most clinical conditions, it is common for rheumatologists to prescribe vitamin D for patients who are suffering from RA [21]. Several studies have been carried out among people in sunny regions such as the Near East and North Africa region. In Middle East countries there was reported to be a high prevalence of vitamin D deficiency [22]. The DAS28 and FACIT-F have been shown to be influenced by the metabolism of vitamin D supplements and increased bone resorption [23]. In the current study, at baseline, no statistically significant differences were observed between the two study groups in terms of the following parameters: age, sex, ESR, Ca, CRP, vitamin D levels, DAS28 score, and FACIT-F (p > 0.05). These findings align with the comparable investigations conducted in India and others in Birmingham [8, 24]. Additionally, two studies were carried out among people among the population in Libya. According to Faid et al. [25], their study confirmed that vitamin D status assessment revealed inadequate levels (25(OH)D < 50 nmol/l) in almost 80% of Libyan women. Another study was conducted by Ahmed et al. [13], in which the prevalence of vitamin D deficiency was estimated at 55.63% among the adult population in the Tripoli region, Libya. It also reported that the means of vitamin D deficiency were 13.93 ±3.46 and 11.32 ±4.16 in males and females respectively. In the current study the mean was lower: 7.93 ±3.84 nmol/l in group A and 7.51 ±4.06 nmol/l in group B.
In a recent study, there was statistically significant difference between fatigue scores and vitamin D supplement (group A) after a 12-week treatment with a p < 0.05. These results were in line with other studies [8, 26]. On the other hand, a significant positive correlation was observed between the levels of vitamin D and the scores obtained on the FACIT-F scale. This result was supported by a study carried out in Saudi Arabia [26]. Furthermore, a study was carried out in India and showed an inverse correlation between vitamin D levels and DAS28 [27]. The previous result agreed with the outcome of the current study, which revealed a weak inverse correlation between DAS28 and vitamin D levels with p > 0.05. Other findings from the current study revealed no statistically significant difference in ESR, CRP, Ca levels, DAS28 and two types of drugs between groups A and group B; however, a reduction in ESR, CRP levels, and DAS28 score and a slight increase in Ca level parameters were noted with p > 0.05. In general, several studies have reported similar results [16, 28, 30]. Finally, there was a significant association between disease activity and FACIT fatigue after intervention of vitamin D in the supplemented group A (r = 0.557, p = 0.000). The previous result was in the same line with another study that was carried out at Mianyang Central Hospital, China [31].
Limitations
There was a relatively short follow-up (12 weeks) due to the lack of refraining from vitamin D supplementation in group B. There was a relatively small study group of RA patients.
It is difficult to compare MTX treatment regimens between RA patients in Libya and those in Europe, because most RA patients in Libya use lower doses with efficacy and without toxicity, as presented in the paper by Etaher et al. [32], compared to the data from European countries [15].
Strengths of the study
It was a homogeneous group with a definite diagnosis of RA and homogeneous Libyan population.
The benefit is to point out that also in populations living in areas of high sunlight there are deficiencies or even deficits of vitamin D in the general population, which additionally intensifies inflammatory diseases such as RA.
Conclusions
The results of the current study indicated that vitamin D3 (50,000 IU of cholecalciferol) had a positive impact in RA patients, compared to the therapy with conventional drugs (DMARDs) without vitamin D supplementation, as was clear from the significant FACIT-F.
However, further research with a larger sample size and longer duration is recommended to confirm and generalize these results.