Introduction
Primary Sjogren’s syndrome (pSS) is an autoimmune disease affecting primarily exocrine (especially salivary) glands. The epithelial damage, autoantigens release, and activation of innate and acquired immunity underlie the pSS pathogenesis. B-lymphocytes and the production of autoantibodies play a key role in pSS. The development of pSS is favored by certain factors, both genetic and environmental, including viral infections, mainly with Epstein-Barr virus, microbiome dysbiosis, ultraviolet radiation (UV), and hormonal imbalance [1].
From the moment the epithelial cells are damaged interferon gamma (IFN-γ) is secreted by cells such as macrophages or dendritic cells, and the cells are stimulated to secrete a B-cell activating factor (BAFF), stimulating the maturation and differentiation of B cells and other cytokines that stimulate B lymphocytes, such as a proliferation-inducing ligand (APRIL), which has a similar effect to BAFF. Mediators of B-cell survival and maturation also include 3 Fms similar tyrosine kinase ligand (FLT-3L) acting through receptors for tyrosine kinase 3 (CD135), which are found on hematopoietic progenitor cells. This cytokine also affects the maturation of dendritic cells and NK cells, activates lymphocyte cytotoxicity, and has anti-tumor activity. FLT-3L is important in the development of plasmacytoid dendritic cells (pDC) and classic dendritic cells (DC) [1].
Tumor necrosis factor α (TNF-α) and tumor growth factor β (TGF-β) are its inhibitory factors [2, 3]. Lymphotoxin α (LT-α), previously named tumor necrosis factor β (TNF-β), is a protein secreted directly by Th1 lymphocytes, unlike TNF-α secreted by macrophages and monocytes. Lymphotoxin α affects the development of a specific immune response at the level of peripheral lymphoid organs, and it has an effect on promoting the production of interferon and chemokines. Shen et al. [4] proved that LT-α, concentration increases mainly locally in salivary glands and in the serum of patients with pSS.
Another important in cytokine network in pSS is IL-21 that increases B cell proliferation, B cell production of immunoglobulins, cytotoxicity and proliferation of NK cells, as well as T-cell proliferation. Its action, however, is also pleiotropic because in the presence of IL-4 it inhibits the proliferation of B lymphocytes. Together with FLT-3L and IL-15, it affects the production of NK cells in the bone marrow and increases their cytotoxicity. It also affects the differentiation of naïve T lymphocytes into Th17 lymphocytes, and synergistically, with BAFF, it promotes B-cell differentiation. It has been proven that IL-21 concentration correlates with severity of salivary infiltrates, while the effect on IL-21 B-cell proliferation increases in the production of immunoglobulins and autoantibodies in PS [5, 6].
Tumor necrosis factor α (TNF-α) is a well-known, mainly proinflammatory cytokine, secreted mainly by macrophages and by TCD4+cells, NK cells, neutrophils, mast cells, and eosinophils, which take part in acute immune response as a immunoregulator, and inflammatory enhancer, which in subsequent stages of inflammatory process is involved in maturation of dendritic cells (DC) and activation of endothelial cells (EC), as well as its chronic stimulation maintaining survival of long-lived plasma cells.
Tumor necrosis factor α is largely responsible for inflammatory systemic symptoms such as fever and cachexia, but also has an inhibitory effect on T lymphocytes, TCR receptors and induce regulatory T cell activation and expression of anti-apoptotic proteins. It is associated with many other pro-inflammatory cytokines like e.g. IL-6, IL-18, IL-1 by means of mutual stimulation as well as inhibition. In some autoimmune diseases, not only its leading role in the inflammatory process has been confirmed but also the effective treatment based on its inhibition (e.g. in rheumatoid arthritis ankylosing spondylitis or psoriatic arthritis) were introduced [7].
Among other proinflammatory cytokines such as IFN-γ and FasL, TNF-α is involved in apoptosis of the salivary glands and lacrimal gland cells in pSS, which leads to destruction of salivary and lacrimal glands [8]. Caffery et al. [9] studied patients with pSS, dry eye, and healthy control, and found that TNF-α expression mRNA was higher in the Sjögren’s syndrome patients than in the other studied groups. Discussing the importance of TNF-α in cancer exceeds the scope of this work.
It is known that TNF-α directly and indirectly interferes with the activity of TGF-β, and these cytokines are contrasted as pro- and anti-inflammatory cytokines as well as anti- and profibrotic. However, their mutual dependencies are still not fully clear, and research is being conducted in the context of the significance of these influences in the pathogenesis of autoimmune diseases and the course of infection.
The presented work focused on TGF-β, which is the one of the anti-inflammatory cytokines, which may play a role in autoimmune processes. This is a pleiotropic cytokine, which is involved in the control of autoimmune reactions, cell proliferation, and the organ accumulation of lymphocytes. The role of TGF-β in the prevention of autoimmune diseases has been presented in the literature, and the development of the autoimmune process has been studied in animal models such as in TGF-β1-deficient mice [10, 11]. TGF-β is also recognized as an important factor in the pathogenesis of fibrogenesis in systemic sclerosis (SSc) [12]. Therefore, in SSc new therapies targeting this cytokine are being sought. However, also in other autoimmune diseases, including Sjögren’s syndrome, its protective effect on the activity of the immune response and the possibility of its use in treatment are still being investigated [13].
Saxena et al. [14] presented research concerning TGF-β and explained that the reduced TGF-β production in immune cells predisposes to immune dysregulation and autoantibody production. The authors also suggested that in organs affected by inflammation the production of anti-inflammatory cytokines, such as TGF-β, is stimulated to counter inflammation. This mechanism acts as a “double-edged sword”, because the enhanced TGF-β production in target organs may lead to progressive fibrogenesis, dysregulated tissue repair, and finally to organ damage. Numerous studies have focused on the expression of TGF-β in salivary glands [15], as organs targeted in pSS, with TGF-β considered not only in a pathogenic context, but – what is interesting – also as a treatment option [16]. Knowledge of the multidirectionality of TGF-β potential in the course of inflammatory rheumatic diseases gave rise to the research presented in this work on patients with pSS.
Objectives
The aim of this study was to evaluate the level of TGF-β in serum of patients with pSS and to show the relationship between TGF-β and the main autoantibodies and cytokines active in the pathogenesis of this syndrome as well as with the presence of cells (assessed with immunochemistry) in specimens from minor salivary gland biopsies.
Material and methods
Thirty-three patients with pSS (diagnosed according to current EULAR/ACR criteria) were included in the study, female 28 (85%), men 5 (15%), mean age 47 ±SD = 16. Routine laboratory tests (blood morphology, ESR, CRP, rheumatoid factor) were performed along with ophthalmological assessment (Schirmer’s test, ocular staining score) with confirmation of dry eye. Patients had routine chest X-ray and, in case of clinical indications, high-resolution computed tomography to assess interstitial changes. In the studied group confirmation of interstitial lung disease (ILD) was confirmed in five patients (15%). Serum concentrations of TGF-β were determined using a Quantikine ELISA Kit. Serum levels of cytokines: BAFF, APRIL, FLT-3L, LT-α, IL-21, and TNF-α were evaluated using standard ELISA assays. The presence and titer of antinuclear antibodies (IF; HEp-2000) was measured. The presence of anti-SS-A and anti-SS-B antibodies was determined by semi-quantitative immunoblotting evaluation. A biopsy of minor salivary glands with histopathological evaluation (focus score – FS) and immunochemistry (CD3+, CD4+, CD19+, CD21+, CD35+ cells) was performed.
The study was approved by the National Institute of Geriatrics, Rheumatology, and Rehabilitation Bioethics Committee.
Results
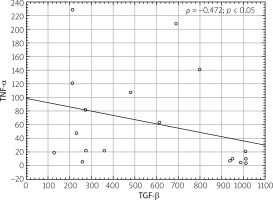
There was no significant correlation between TGF-β and other tested cytokines or autoantibodies (ANA, anti-SS-A, anti-SS-B), other than TNF-α. The obtained results are summarized in Table I. The negative correlation (ρ = –0.472) between TGF-β and TNF-α is shown graphically in Figure 1. There were no correlations between TGF-β and: results of ocular examinations, FS, and biopsy examination using immunochemistry. There was no correlation between TGF-β and lung involvement, especially lung fibrosis, in this group. Also, no other correlation of TNF-α with other variables – apart from the above-mentioned correlation with TGF-β – was demonstrated.
Table I
Correlations of TGF-β with measured parameters
[i] TGF-β – tumor growth factor β, WBC – white blood cells, ESR – erythrocyte sedimentation rate, ASPAT – aspartate aminotransferase, ALAT – alanine aminotransferase, CRP – C-reactive protein, TSH – thyroid-stimulating hormone, ACPA – anti-citrullinated protein antibodies, OSS – ocular staining score, ANA – antinuclear antibodies, BAFF – B-cell activating factor, APRIL – a proliferation inducing ligand, FLT-3L – 3 Fms similar tyrosine kinase ligand, LT-α – lymphotoxin α, TNF-α – tumor necrosis factor α
Discussion
The negative correlation between TGF-β and TNF-α confirms the anti-inflammatory properties of TGF-β. Many studies have shown that the roles of both cytokines are opposite to each other [2, 11, 12].
Paradoxically, it has been widely underappreciated that with the inhibition of TNF-α in the treatment of some rheumatic diseases, e.g. rheumatoid arthritis, the TNF-α/TGF-β signaling pathway is also inhibited. As the inhibition of TGF-β by TNF is being blocked, it results in an increased production of TGF by macrophages. This protective role of TGF-β in the development of active inflammation is attributed to its activity as a strong natural TNF-α inhibitor. This suggests that stimulating TGF-β production can be used as a potential therapy method [17].
Tumor transforming growth factor-β also plays a crucial role in the process of tissue repair. It enhances collagen I gene expression, in contrast to TNF-α, which reduces collagen type I gene expression [18]. The interplay between these two factors allows the stability of functions to be maintained as well as the integrity of organs and tissues; it also helps to maintain proper regeneration and repair [18]. However, the overstimulation of the production of extracellular matrix by TGF-β may lead to a disbalance between normal regeneration and fibrosis. In this way TGF-β may act as a pro-fibrotic cytokine [2, 18]. Importantly, this cytokine has been shown to be an important factor in pathological fibrogenesis in SSc – regulating cell growth, their differentiation, apoptosis, and above all, the synthesis of extracellular matrix [12].
The obtained results, although from a small group of patients with pSS, emphasize that both of these cytokines are present in serum patients with pSS and confirm their interdependence. This interdependence may underlie failures of anti-TNF-α therapy in pSS, reported in prior studies [19, 20], as well as the risk of developing interstitial changes, leading to pulmonary fibrosis [21], which has been associated with TGF-β activity. There was no direct correlation between the TGF-β level and interstitial lung changes in the study group, but the group of patients with ILD (n = 5) is far too small to be able to rely on such an analysis.
Mieliauskaite et al. [22] analyzed patients with RA and secondary Sjögren’s syndrome and concluded that the level TGF-β positively correlates with the development of arthritis and with bone destruction in RA. This phenomenon is surprising taking into account the recognized anti-inflammatory properties of this cytokine. The authors described no significant relationship between TGF-β activity and the occurrence of secondary Sjögren’s syndrome.
Interestingly, Loubaki et al. [23] demonstrated that a high dose of intravenous immunoglobulins (IVI g) increases the expression of TGF-β in monocytes and mediates its tolerogenic effect. The authors also studied indoleamine 2,3-dioxygenase (IDO) – a protein-enzyme produced in particular by dendritic cells and macrophages after their stimulation with either TGF-β, IFN, or LPS. Among other properties, IDO may act as a signaling transducer and confer a tolerogenic phenotype to plasmacytoid DCs (pDCs). This enhances the production of TGF-β and affects T regulatory cells [24, 25].
In scientific research and clinical trials, the concept of novel therapies targeting modulating anti-inflammatory signaling pathways, such as with TGF-B, are being investigated [26]. Montelone et al. [27] investigated in patients with Crohn’s disease mongersen (GED0301) antisense oligonucleotide, which is an inhibitor of SMAD7 – which in turn in vivo reduces activity of TGF-β1 in the course of this bowel inflammatory disease. Preclinical studies and a phase 1 study have shown that such treatment restores the anti-inflammatory effects of TGF-β in these patients. It proves that the use of anti-inflammatory cytokine-activating pathways, alongside the already widely used pro-inflammatory cytokine inhibitors, creates potential new directions in the treatment of inflammatory autoimmune diseases, including Sjogren’s syndrome. On the other hand, due to the confirmed profibrotic abilities of TGF-β and its role in fibrogenesis in SSc, the inhibition of this cytokine may also be a therapeutic target [28, 29].
Such opposing views and searches lead to the conclusion that each autoinflammatory disease, including Sjögren’s syndrome, should be considered separately in the context of the role of TGF-β and the possibility of therapy, which can be directed in different ways.
Limitations of the presented study
The relatively small size of the studied group, especially considering the evaluation of clinical aspects of the disease, such as lung fibrosis, is the main limitation of the present work. However, the study group was selected to represent all relevant immunological features (autoantibodies), and histopathological (focus score) and clinical (dryness) characteristic for primary Sjögren’s syndrome.
The fact that the results concerned only patients with diagnosed primary Sjögren’s syndrome may be considered as another limitation of our research. However, the study was strongly focused on demonstrating the relationship between the studied parameters in pSS patients.