Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that manifests with a variety of clinical symptoms and abnormalities in laboratory tests. The course of the disease is progressive and can lead to irreversible organ damage and death of the patient [1]. Early diagnosis of SLE and effective treatment tailored to disease activity are important in improving prognosis. A well-conducted activity assessment makes it possible to distinguish between SLE symptoms resulting from its exacerbation and chronic damage, which may be a consequence of the disease itself, comorbidities or the treatment used [2, 3]. The current approach to treating SLE patients is the treat-to-target strategy (T2T), commonly used in rheumatic diseases. A key element of this strategy is defining the treatment goal and reliably assessing SLE activity, which will allow good therapy planning and consequently achieving the desired goal of remission or low disease activity [4]. Various tools have been developed to assess activity and organ damage in SLE patients, including the British Isles Lupus Assessment Group (BILAG) scale, the Easy-BILAG, the European Consensus Lupus Activity Measurements (ECLAM), the Systemic Lupus Activity Measure (SLAM), the SLE Disease Activity Index (SLEDAI), the SLE Disease Activity Score (SLE-DAS), the Physician Global Assessment (PGA) and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI) [5–7]. Based on selected activity scales, criteria for low SLE activity – the Lupus Low Disease Activity State (LLDAS) criteria, and disease remission – the Definitions Of Remission In SLE (DORIS) criteria, have been established [8–10].
Systemic Lupus Erythematosus Disease Activity Index and its modifications
First published in 1992, the SLEDAI scale has become a common tool for assessing SLE activity. Several modifications of this scale have been developed over the past few years, including: the SLE Disease Activity Index 2000 (SLEDAI-2K), the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA-SLEDAI), and the Mexican Modification of the Systemic Lupus Erythematosus Disease Activity Index (Mex-SLEDAI) [11–13]. One of the most widely used modifications is the SLEDAI-2K scale, developed in 2002. The scale’s questionnaire contains 24 clinical symptoms, of which 16 are clinical and 8 are based on laboratory findings. A given symptom is considered present if it occurs regardless of its severity. In the original version of the SLEDAI, skin symptoms, mucosal ulcers and proteinuria were considered active only if they occurred for the first time or recurred. SLEDAI-2K scores points for the presence of rash, alopecia, mucosal ulcers and proteinuria > 0.5 g/day and also when they persist chronically. The patient receives points if the symptom appeared in the last 10-30 days. A maximum of 105 points can be obtained (Table I) [14].
Table I
SLEDAI-2K descriptors and scores [14]
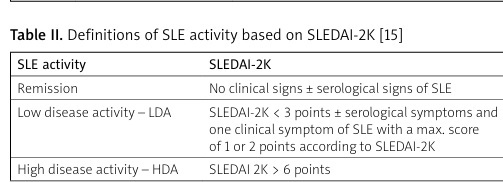
Based on SLEDAI-2K, the definition of remission, low disease activity (LDA) and high disease activity (HDA) was established. Remission is considered the absence of clinical symptoms of SLE (with or without serological signs), in patients without treatment or taking antimalarial drugs. Low disease activity is considered SLEDAI-2K < 3 (with positive or negative serological tests) with only one clinical sign present, with a score range of 1 to 2, in patients taking antimalarials (without glucocorticosteroids [GCs] and other immunosuppressants). High disease activity is a SLEDAI 2K score > 6 (Table II) [15].
Table II
Definitions of SLE activity based on SLEDAI-2K [15]
According to Carter et al., one can divide SLE activity based on SLEDAI-2K into severe (SLEDAI-2K > 12), moderate (6 < SLEDAI-2K ≤ 12), mild (0 < SLEDAI-2K ≤ 6) and remission (SLEDAI-2K = 0) [16]. A clinical variation of the SLEDAI scale in clinical practice (clinical SLEDAI-2K – cSLEDAI-2K) is being used increasingly. It does not take into account the results of serological tests (concentration of anti-dsDNA, C3 and/or C4 complement) but does take into account the use of antimalarial drugs, low doses of GCs, and immunosuppressive drugs, including biologics. In some SLE patients, immune activity may persist for a long time despite clinical inactivity. According to many researchers, such a condition does not increase the risk of SLE exacerbation [17–20].
Values obtained on the SLEDAI-2K scale are a measure of total SLE activity and a good predictor of mortality. Its significant advantage is the facility and speed of filling, which allows it to be widely used in clinical settings. Disadvantages of this scale may include: a fixed score of individual SLE symptoms (a patient with thrombocytopenia of 50,000/μl receives 1 point, as does a patient with a score of 3,000/μl), inability to assess the degree of improvement or worsening of clinical symptoms, and failure to take into account other symptoms of SLE, including hemolytic anemia, pneumonia and gastrointestinal symptoms [14, 21].
The SELENA-SLEDAI scale is applicable to premenopausal women taking oral contraceptives and postmenopausal women taking hormone replacement therapy. The SELENA-SLEDAI assesses the same 24 clinical symptoms as in the original SLEDAI scale that are present at the time of the visit or within the past 10 days. The SELENA-SLEDAI (like the SLEDAI-2K) also takes into account chronically persistent skin lesions [22, 23].
The Mex-SLEDAI scale was developed by Mexican researchers in 1992. It is a simplified version of the original SLEDAI scale. Some clinical manifestations of SLE, such as fatigue and lymphopenia, were added to it and others (lupus headache and visual disturbances) were removed. The Mex-SLEDAI scale does not take into account the results of laboratory tests, i.e. complement components or anti-dsDNA antibodies. A maximum score of 32 can be obtained and a score ≥ 5 indicates active disease. The sensitivity of this scale is 87.5% and specificity is 100%. Evaluation of SLE activity using Mex-SLEDAI is significantly less expensive compared to the classic SLEDAI scale [13].
General assessment of disease activity by the doctor – Physician Global Assessment
The PGA scale is a tool for estimating overall disease activity that is intended to allow the physician to present information about disease activity found at the time of the current evaluation using a Visual Analogue Scale (VAS).
The evaluation should be carried out by a physician, taking into account clinical activity, the functioning of various organs and systems, values of laboratory indicators and radiological data [24].
The activity determined by the PGA includes the following:
The PGA scale has high reliability when used by rheumatology specialists. It is sensitive to changes in activity and comprehensively takes into account all aspects of the disease. The PGA has been shown to be associated with permanent damage arising in the course of SLE. Disadvantages of PGA include the subjectivity of assessment and lack of standardization.
Despite its drawbacks, the PGA is rated as a good tool for assessing changes in disease activity and a good indicator of disease exacerbation. The PGA scale is incorporated into various systems for assessing the response to therapy such as DORIS and LLDAS.
Systemic Lupus Erythematosus Disease Activity Score
An increasingly used tool for assessing SLE activity is the SLE-DAS index, which is based on the SLEDAI-2K scale. It was developed and validated by Jesus et al. in 2019. These researchers conducted a cohort study involving 520 SLE patients. Disease activity was assessed using the SLEDAI-2K scale and PGA, which was the dependent variable in the SLE-DAS construct. This indicator was validated in another cohort, taking into account the correlations occurring between PGA, SLEDAI-2K and SLE-DAS. The problem in assessing disease activity in the scales available to date has been the inability to distinguish between disease remission and LDA. Therefore, it was proposed to include a physician’s assessment of PGA disease activity in conjunction with laboratory results and clinical evaluation. The study showed a strong correlation of SLE-DAS with PGA (r = 0.875, p < 0.0005) and SLEDAI-2K (r = 0.943, p < 0.0005). The SLE-DAS had higher sensitivity in detecting significant clinical improvement or worsening compared to the SLEDAI-2K scale (89.5% vs. 47.4%, p = 0.008 and 95.5% vs. 59.1%, p = 0.008, respectively), as well as a higher predictive value of damage accrual. An important factor contributing to this difference was the use of ongoing measurements of arthritis severity, proteinuria, thrombocytopenia and leukopenia in the SLE-DAS. A limitation of the above study was PGA, which was a subjective assessment by the physician. In the SLE-DAS, compared to the SLEDAI-2K, the number of scored symptoms was reduced from 24 to 17 and clinically significant symptoms were added, including: hemolytic anemia, gastrointestinal disorders, peritonitis, and cardiac/lung involvement (Table III) [25, 26]. This seems particularly relevant in the male population, which is more likely to have cardiac involvement and hemolytic anemia in the course of SLE [27]. Subsequent studies have confirmed a significant correlation between SLEDAI-2K and SLE-DAS. In patients with low SLE activity, the SLE-DAS index showed higher sensitivity and specificity compared to SLEDAI-2K [25, 28].
Table III
The SLE-DAS: clinical and laboratory parameters attributable to SLE [25]
The SLE-DAS scale is distinguished by the variability of the value of an indicator depending on the degree of disease activity, which allows for more precise monitoring of SLE activity. This includes symptoms such as arthritis, proteinuria, leukopenia and thrombocytopenia. A complex mathematical formula is used to calculate the SLE-DAS, and in clinical practice the use of a calculator is essential [29]. The advantage of the SLE-DAS index is its easy availability (online) and the short time it takes to conduct an assessment of disease activity. A limitation of the SLE-DAS is the assessment of renal parameters based on proteinuria only; SLEDAI-2K additionally considers sterile leukocyturia, hematuria, urinary casts and fever.
Based on SLE-DAS values, the following categories of SLE activity were developed: remission, mild, low and moderate/severe disease activity (Table IV). Disease activity categories in the SLE-DAS are easy to define and only require an SLE-DAS assessment during an ongoing medical visit. The sensitivity and specificity of using the SLE-DAS to assess remission, mild and moderate/severe SLE activity were 90%, 82% and 95%, respectively [30, 31].
Table IV
SLE-DAS disease activity categories [30]
| SLE-DAS disease activity category | Disease activity |
|---|---|
| SLE-DAS > 7.64 | Moderate/severe disease activity |
| 2.08 < SLE-DAS ≤ 7.64 | Mild disease activity |
| SLE-DAS ≤ 2.48 | Low disease activity |
Assessment of SLE activity in pregnant women
A modification of the SLEDAI scale used to assess SLE activity in pregnant women is the Systemic Lupus Erythematosus Pregnancy Disease Activity Index (SLEPDAI) [32]. Pregnancy is known to lead to physiological changes that can be interpreted in pregnant women with SLE as symptoms of an exacerbation of the autoimmune disease (e.g., edema, proteinuria, skin lesions). The SLEPDAI scale assesses the same clinical and immunological parameters as the basic variant of the SLEDAI scale, but attention should be paid to physiological changes in the pregnant woman’s body that may resemble symptoms of SLE exacerbation (Table V) [33].
Table V
SLEPDAI descriptors [33]
A high SLEPDAI score correlates with an increased risk of complications in a pregnant SLE patient and her child. There was a significant correlation between SLEPDAI and the occurrence of preeclampsia/pregnancy eclampsia, preterm labor, and low neonatal birth weight (Table VI) [34–37]. The SLEPDAI scale has not yet been fully validated, and further research is needed to confirm its reliability. The SLE-DAS is also used to assess SLE activity in pregnant women. Larosa et al. assessed SLE activity in pregnant women during the first trimester of pregnancy using the SLE-DAS and SLEPDAI. They found a strong correlation between these indicators (r = 0.97, p < 0.01). Both of these scales also predicted the appearance of complications at later stages of pregnancy [38]. Both the SLEPDAI and SLE-DAS scales are simple and effective in assessing SLE exacerbations in pregnant women and in predicting pregnancy complications. It appears that SLE-DAS may be preferable due to its ease of use and continuous evaluation capabilities.
Table VI
Relationships between SLEPDAI and pregnancy complications in women with SLE – results of selected retrospective studies [34–37]
| Author | Results of the study |
|---|---|
| Çetin et al. [34] | Higher SLEPDAI score correlated with increased risk of fetal/neonatal death, premature labor due to pre-eclampsia/pregnancy eclampsia, HELLP syndrome, low neonatal birth weight |
| Murata et al. [35] | SLEPDAI > 4 significantly correlated with risk of premature labor and pre-eclampsia |
| Erazo-Martínez et al. [36] | Higher SLEPDAI score significantly correlated with the risk of pre-eclampsia and eclampsia of pregnant women |
| Ignacchiti Lacerda et al. [37] | SLEPDAI ≥ 4 correlated with low birth weight of newborns |
Use of activity scales to assess remission and low systemic lupus erythematosus activity
The goal of SLE treatment is to achieve remission or low disease activity. In 2016, Franklyn et al. presented a definition of the lupus low disease activity state – LLDAS of SLE [39]:
SLEDAI-2K ≤4 points, with the absence of activity in major organs/systems (kidney, central nervous system, cardiopulmonary system, vasculitis, fever) and the absence of hemolytic anemia and active gastrointestinal inflammation.
No new signs of disease activity compared to previous examination.
PGA value < 1.
Current dose of prednisone or equivalent dose of other GCs ≤ 7.5 mg/day.
Good tolerance of maintenance doses of immunosuppressant or biologic drugs.
In 2016, as part of the operation of the so-called DORIS initiative, a definition of remission in SLE was established [40]. The current, updated version of the DORIS criteria is from 2021 and includes the following criteria:
SLEDAI-2K = 0.
PGA < 0.5.
Good tolerance of treatment with antimalarial drugs, prednisone < 0.5 mg/day or equivalent, and/or an immunosuppressant or biologic [41].
Both definitions use the SLEDAI-2K scale score, demonstrating the importance of this scale in assessing prognosis and treatment planning. Pawlak-Buś et al. found that the strongest predictor of remission according to DORIS and LLDAS was the mean SLEDAI-2K score. The lower the SLEDAI-2K value was, the higher was the chance of achieving remission or low disease activity. The authors found that in untreated SLE patients, SLEDAI-2K ≤ 8 was a significant predictor of DORIS and LLDAS goal achievement (p < 0.001 and p < 0.001, respectively). In treated patients, a SLEDAI-2K value ≤ 12.5 was the most important predictor of these targets (DORIS p = 0.004 and LLDAS p = 0.002) [42]. In clinical practice, the evaluation of SLE treatment efficacy should be measured with an easy and objective tool. According to Saccon et al. the goal of SLE treatment is to achieve SLEDAI-2K = 0. This value correlates with a low probability of organ damage in remission lasting at least 2 years, regardless of the PGA value. The authors noted that achieving SLEDAI-2K = 0 preceded the achievement of PGA < 0.5. This was likely due to the presence of symptoms such as fatigue or joint pain, which affected PGA but did not affect SLEDAI-2K values or organ damage [43].
Two criteria for remission have been proposed, based on the SLE-DAS scale score. The first criterion defines SLE remission in patients with SLE-DAS ≤ 2.08 taking prednisone ≤ 5 mg/day. The second criterion is based on the Boolean index: achieving a score of 0 in all SLE-DAS clinical domains and a daily dose of prednisone ≤ 5 mg. The criterion for remission based on the SLE-DAS index value is a cumulative score and reflects overall disease activity, thus giving some flexibility in assessing individual symptoms. The Boolean index is more stringent, requiring low values in each test domain present at the same time. Both SLE-DAS remission criteria were found to be comparable to DORIS clinical remission criteria. The SLE-DAS has the advantage of being easy to use – it does not require PGA or specific recommendations for antimalarials, immunosuppressants or biologics [26]. Remission according to the SLE-DAS shows 100% concordance with DORIS criteria. Low SLE activity according to the SLE-DAS (SLE-DAS ≤ 2.48 and prednisolone dose ≤ 7.5 mg/day) showed more than 97% agreement with LLDAS. The criteria for remission and LDA based on the SLE-DAS index are easier to apply compared to the DORIS and LLDAS definitions [44].
However, some researchers consider that a more effective tool for LDA and SLE remission is the SLE-DAS rather than the SLEDAI scale. Assunção et al. reported that a certain percentage of those meeting LLDAS criteria still had active arthritis (1%), skin lesions (1.4%) or mucosal ulcers (0.4%). None of these individuals met the LDA criterion of the SLE-DAS, which would suggest the greater sensitivity of this scale in assessing low disease activity [45]. Cunha et al. found that as many as 7.5% of SLE patients with SLEDAI ≤ 4 did not reach the LDA criterion according to the SLE-DAS [46]. Shumilova et al. in a study of 228 SLE patients found that the SLEDAI-2K scale had higher specificity than the SLE-DAS for assessing SLE remission (89% and 79%, respectively), but the SLE-DAS was more specific than the SLEDAI-2K for assessing low disease activity (80% and 59%, respectively) [47].
The SLE-DAS has been shown to have some advantage over the SLEDAI-2K in assessing the risk of hospitalization for SLE and other causes. A prospective cohort study involving 326 Taiwanese patients showed that SLE patients with moderate/severe disease activity according to the SLE-DAS had a significantly higher risk of hospitalization for SLE as well as for other causes. The SLEDAI-2K value showed no significant correlation with the risk of hospitalization for SLE exacerbation and only a slight association with hospital admissions for other reasons [48]. Similarly, Wang et al. found that patients with moderate/severe SLE activity according to the SLE-DAS index were more likely to be hospitalized for both general and SLE-related causes. Moderate or severe activity according to SLEDAI-2K was only significantly associated with hospitalization of SLE patients for general causes [49]. This difference can be explained by the inclusion of heart/lung involvement in the course of SLE in the SLE-DAS compared to SLEDAI-2K.
Summary
Reliable assessment of SLE activity is key to making treatment decisions. In clinical practice, we particularly often use the SLEDAI-2K scale. More and more data are confirming the usefulness of the SLE-DAS index. Both tools are of comparable value in assessing SLE activity and complement each other.