Introduction
Objectives
Systemic lupus erythematosus (SLE) is a generalized autoimmune disease in which diffuse chronic inflammation plays an important role. Today, survival in patients with SLE is limited by the increased occurrence of serious cardiovascular complications [1].
Risk factors for coronary artery disease (hypertension, hyperlipidaemia, diabetes, tobacco use, and sedentary lifestyle) do not explain the increased risk of atherosclerosis and cardiovascular complications in patients with SLE [2]. One of the possible mechanisms includes chronic, generalized inflammation, as well as the presence of antiphospholipid antibodies (aPL). Elevated levels of aPL are associated with an increased risk of thrombosis in the arteries and microcirculation [3, 4]. This co-existence meets the criteria for the diagnosis of antiphospholipid syndrome (APS) [5], which occurs in one-third of patients with SLE [6].
Non-invasive methods of measuring vascular parameters are used to assess the risk of development and progression of cardiovascular diseases. The loss of elasticity of the arterial walls, in particular the aorta, is believed to be a marker of early changes that may lead to the development of atherosclerosis and a wide spectrum of its complications (e.g. myocardial infarction, ischaemic stroke, limb ischaemia) [7]. Pulse wave velocity (PWV) is used to detect an early increase in aortic wall stiffness and is valued by the European Society of Cardiology as a marker of early atherosclerotic damage [8].
There is abundant evidence in the literature of a high predictive value of carotid-femoral pulse wave velocity (cfPWV) in the evaluation of the risk of cardiovascular complications in the elderly [9], in patients with arterial hypertension [7], type 2 diabetes [10] or end-stage renal disease [11]; cfPWV also allows independent evaluation of cerebrovascular risk in healthy subjects [12].
Von Willebrand factor (vWF), produced by the endothelium, plays a role in platelet activation and aggregation. Its blood concentration increases in endothelial damage, especially in diseases that accelerate atherosclerosis, such as arterial hypertension, hyperlipidaemia, and nicotinism [6, 13]. Although elevated blood vWF concentration can also be found during pregnancy and after physical activity in otherwise healthy subjects, vWF is considered a good marker of endothelial damage [14, 15]. The general advantage of vWF as an indicator of endothelial damage is that it is produced only by the endothelium and its level remains stable for weeks [14].
Thrombin–antithrombin III complexes (TAT) are formed when thrombin, generated by thrombotic pathways, is inactivated by circulating antithrombin III. High levels of TAT are associated with high prothrombotic activity and may be found in clinical conditions such as myocardial infarction, atrial fibrillation, or pulmonary embolism [16]. On the other hand, chronic anticoagulation leads to a decrease in serum TAT levels [16]. Thrombin–antithrombin III complex represents a short-lived marker of prothrombotic activity and is widely used in studies on coagulation disorders [6].
The increase in aortic stiffness assessed with cfPWV well reflects the increased risk of cerebrovascular and cardiovascular diseases [8]. Although vessel wall stiffening is primarily related to ageing, many studies have shown an acceleration in vascular stiffness in patients with risk factors for cardiovascular disease [7]. Changes in the structure of the vessel wall become the basis for the development of atherosclerosis, which, as an inflammatory process, is also intensified in systemic inflammatory diseases [17].
Material and methods
This prospective single-centre study was carried out in 26 consecutive patients treated for SLE in the Department of Internal Medicine of the Jagiellonian University Medical College, Krakow. All patients met at least 4 ACR classification criteria for SLE [18].
Inclusion and exclusion criteria
The inclusion criterion was a stable clinical condition of SLE (no need to intensify immunosuppressive therapy, that is, an increase in current doses of immunosuppressive drugs or the introduction of an additional immunosuppressive drug in the last three months).
Exclusion criteria were the following: a history of coronary artery disease, known cancer, clinical symptoms of heart failure (New York Heart Association III or IV class), renal failure (creatinine clearance < 30 ml/min) and/or respiratory failure.
Pulse wave velocity
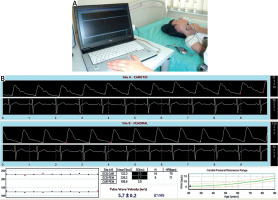
Pulse wave velocity was measured transcutaneously in the right common carotid artery and in the right femoral artery independently, using SphygmoCor. The applanation tonometer (Millar®) was applied first to the common carotid artery and then to the femoral. The measurement of PWV was performed simultaneously with the ECG examination (Fig. 1 A). The propagation time is expressed as the difference between the beginning of pulse waves in both arteries and the contraction of the left ventricle, represented by the wave R in the ECG. This method neglects the fact that the wave in both arteries comes from two separate cycles of the heart cycle. It is believed that with a sufficiently short period of time between both measurements, possible changes in heart rate or isovolumetric left ventricular contraction time do not significantly affect pulse wave velocity and are negligible (Fig. 1 B) [19].
Fig. 1
Pulse wave velocity measurement. An applanation tonometer (Millar®) is applied to the common carotid artery (A). The pulse wave graph is recorded by the SphygmoCor®, simultaneously with the ECG examination (B).
Pulse wave velocity (PWV) is defined as the ratio of a distance (s) between test points to the time (Δt) needed to travel this distance (PWV = s/Δt). The space between the two measurement points was evaluated above the body surface with a measuring tape and adjusted to the precise vascular anatomy [8]. Pulse wave velocity is expressed in metres per second (m/s). The average of three consecutive measurements was adopted as the final result.
Coronary calcium score
Coronary calcium scoring was performed using a multidetector computed tomography scanner (Somatom Definition, Siemens, Germany). Images were ECG-triggered with 3-mm thick sections covering the whole heart. Coronary artery calcifications were defined as lesions with attenuation greater than 130 Hounsfield units (HU) in more than four adjacent pixels. To quantify coronary calcium, the 3D Leonardo application (Siemens, Germany) was used. The number of atherosclerotic plaques and their volume in particular coronary arteries were evaluated. The Agatston calcium score was calculated [20].
Laboratory tests
Routine laboratory investigations were measured using standard methods. Serum levels of anticardiolipin (aCL) and anti-β2-GPI antibodies (of both IgG and IgM classes) were measured using a homemade ELISA with the Sapporo standard for the measurement of anti-β2-GPI antibodies (HCAL for IgG, EY2C9 for IgM) [13].
Values exceeding the 99th percentile of a healthy population sample were considered positive, that is, > 20 RU/ml for aCL IgG; > 30 RU for aCL IgM; > 3 RU/ml for anti-β2-GPI IgG and > 3 RU/ml for anti-β2-GPI IgG.
The presence of lupus anticoagulant (LA) was tested in accordance with the three step procedure recommended by the International Society on Thrombosis and Haemostasis [21].
Thrombin–antithrombin III complexes and vWF levels were assessed in citrate plasma using commercial kits (Siemens, Germany). The detailed assay methodology was in accordance with the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using the Statistica Six Sigma software. The study was powered to have an 80% chance of detecting a 10% difference in PWV at the 0.05 significance level. To demonstrate such a difference or greater, 12 subjects or more were required in each group based on the values of a previous study [22].
All numerical data were expressed as mean values ± standard deviations for the normal distribution or as the median and interquartile range (IQR) for the non-normal distribution. Continuous variables were compared using the t-test. The χ2 test was used to examine the differences in proportion. The level of statistical significance was predetermined at p < 0.05.
Results
Characteristics of the patients
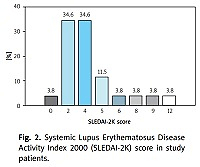
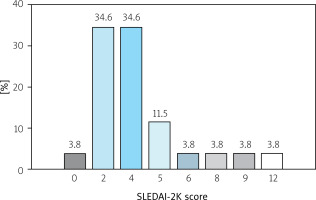
Our study included 26 patients (among them n = 23, 88% women), mean age 39.1 ±11.7 years. The mean duration of the disease at the time of examination was 15.5 (range 2 to 32 years). The severity of SLE according to the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score [23] is shown in Figure 2.
Two patients had previously been diagnosed with APS according to the revised APS classification criteria [5]. One of the two had a history of pulmonary embolism. There were two patients with arterial hypertension and a history of smoking. The results of peripheral blood count, serum sodium, potassium, glucose, creatinine, and urine, as well as ECG recordings at rest, were normal in all patients.
Immunosuppressive treatment included: methylprednisolone (n = 14, 53.8%; 4 mg to maintain clinical stability), prednisone (n = 2, 7.7%), chloroquine derivative (n = 3, 11.5%), azathioprine (n =2, 7.7%), cyclophosphamide (n = 1, 3.8%) and methotrexate (n = 1, 3.8%). The remaining patients did not use any immunosuppressive drugs in the last 12 months of observation.
Other pharmacotherapy included angiotensin-converting enzyme inhibitors in 3 (11.5%) subjects, a β-receptor antagonist in 1 (3.8%) and a calcium channel antagonist in 1 (3.8%). Antiphospholipid syndrome patients were treated with anticoagulant (warfarin, n = 1) or antiplatelet therapy (aspirin, n = 1).
Coronary artery calcium score
Multidetector computed tomography revealed coronary calcifications in 8 (30.8%) patients. The median number and volume of atherosclerotic calcified plaques were 3 (2–23) and 27.6 mm3 (4–761.8 mm3), respectively. The calcium score (CACS) was 52.4 (2–843.2).
Thrombotic biomarkers
The TAT level was 3.7 ±3.5 μg/l, vWF level 143.0 ±47.5 IU/dl, the D-dimer level was 0.73 ±0.92 mg/l. Lupus anticoagulant was present in 5 (19.2%) patients. The concentration of anticardiolipin autoantibodies was as follows: IgM 13.9 ±12.1 RU/ml and IgG 12.3 ±9.0 RU/ml. Regarding anti-β2-GPI antibodies, IgM and IgG levels were 2.2 ±4.3 RU/ml and 1.4 ±1.3 RU/ml, respectively.
Pulse wave velocity
The mean PWV was 9.0 ±3.2 m/s. Pulse wave velocity was higher in patients older than 50 years, those with positive LA, TAT ≥ 10 μg/l, and vWF ≥ 200 IU/dl and with coronary atherosclerosis (CACS > 0). In contrast, disease duration, D-dimers and anticardiolipin and anti-β2-GPI antibodies levels did not influence the PWV (Table I).
Table I
Influence of coronary artery calcium score and thrombotic biomarkers on pulse wave velocity (PWV) in patients with SLE (n = 26)
In the group without atherosclerosis (CACS = 0, n = 18), patients with vWF ≥ 200 IU/dl had 26.8% higher PWV compared to the rest (Table II). We did not observe an association between PWV and other thrombotic markers in this subgroup.
Discussion
Our study confirms that PWV is related to subclinical coronary atherosclerosis in patients with SLE. Furthermore, we observed increased arterial stiffness in patients with positive lupus anticoagulant and elevated TAT and vWF.
Pulse wave velocity in patients with SLE included in our study was 9.0 ±3.2 m/s. The results are similar to those reported in the literature [24, 25]. In a study by Yildiz et al. [24] conducted among young women with SLE and mean age 33.6 ±9.6 years, similar values of pulse wave velocity were obtained (8.98 ±2.05 m/s). Furthermore, Kocabay et al. [25] reported that 22 patients with newly diagnosed SLE aged 35 ±2.5 years achieved an average PWV value of 9.3 ±2.5 m/s.
In the study by Bjarnegråd et al. [26] conducted among 27 women with SLE at an average age of 60 years, women with SLE had a higher pulse wave velocity (9.8 m/s) than patients in the age-matched control group (8.2 m/s), which confirms the influence of age on arterial stiffness, as was also observed in our study.
Our findings on increased arterial stiffness in patients with SLE are consistent with the results of the recent meta-analysis of 18 studies among 943 patients with SLE [27].
As the body ages, significant morphological and functional changes occur in the arterial walls, especially in the middle (media) and subendothelial (intima) layers. Endothelial function is impaired, leading to a reduction in the bioavailability of vasodilating nitric oxide [17]. Disturbances in its production result in an increase in the resting tone of vascular smooth muscle cells, then lead to an increase in arterial stiffness, and finally cause an increase in blood pressure. Additionally, elastin fibres gradually fragment and disappear. They are replaced by more rigid, and thus less prone to stretching, collagen fibres. The amount of intercellular matrix increases and calcium deposits accumulate [17].
Mitchel et al. [9] conducted PWV studies in a population without risk factors for cardiovascular diseases. They divided the study group into four groups according to age (< 50, 50–59, 60–69, > 70 years) and found a significant increase in PWV with age.
An interesting study by Sutton-Tyrrell et al. [28] showed that in older patients (the age of the study group was > 70 years), increased PWV was strongly associated with the occurrence of adverse cardiovascular events and increased mortality.
In our study, we observed a positive correlation between PWV and the presence of coronary calcifications. A higher PWV was found in patients with coronary atherosclerosis (10.2 ±5.2 m/s; +21.4%) compared to patients without coronary calcifications. A similar correlation was previously reported in patients without SLE. Kullo et al. [29] found that PWV was significantly associated with the presence and quantity of coronary artery calcium in patients without a history of myocardial infarction or stroke, while in the study by Liu et al. [30], PWV correlated well with coronary atherosclerosis in a large group of 654 participants.
Modification of lifestyle was the therapeutic intervention that prevented the progression of arterial stiffness. It was also reported that the more advanced ischaemic heart disease was, the higher the PWV values were [22]. In the study of 92 patients who qualified for invasive coronary diagnostics, it was found that PWV was significantly higher in patients with coronary artery lesions (11.13 ±0.91 m/s in single vessel disease, 15.22 ±1.11 m/s in twovessel disease and 19.30 ±2.05 m/s in threevessel disease) than in subjects with normal coronary vessels (8.14 ±1.25 m/s) [22].
The correlation of arterial stiffness, as a marker of early atherosclerosis, with the severity of systemic inflammation has been studied [31, 32], but the studies are not consistent. Blann et al. [31] found that arterial stiffness was not related to inflammation. But his research was conducted among patients with stable coronary artery disease.
On the other hand, Tuttolomondo et al. [32] detected a positive relationship between PWV and some circulating mediators (e.g. vWF) in patients with an acute cardiovascular or cerebrovascular event. In our study, we examined patients with higher levels of inflammatory markers caused by general inflammatory diseases, probably the reason for our positive results.
In patients with SLE, traditional risk factors for atherosclerosis may predict an increased risk of cardiovascular events. However, they do not significantly explain the accelerated progression of atherosclerosis in this group. The primary contribution to both the initiation and progression of atherosclerosis has been attributed to a malfunctioning immune system and, more specifically, to intensified autoimmune responses [2]. This concept was confirmed by Mendoza-Pinto et al. [27], who demonstrated that inflammatory markers influence arterial vessel stiffness in patients with SLE.
The high level of prothrombotic activity in SLE was also described as an important factor that contributes to an increased risk of cardiovascular events [15]. It is suggested to be associated with endothelial damage and autoinflammation [27].
It appears that the rapid progression of atherosclerosis, a result of the pro-inflammatory state in rheumatic disorders, may also increase the probability of thrombotic events. Many studies have shown a correlation between prothrombotic activity and endothelial dysfunction, one of the early stages of atherosclerosis [15, 33]. Thrombin formation has been shown to increase matrix metalloproteinase (MMS) enzymatic activity, contributing to the stiffness of the extracellular matrix of arteries [34].
Furthermore, the degradation of the extracellular matrix by MMS remains a link between arterial vessel remodelling, arterial stiffness, atherosclerotic plaque progression, and destabilization [35]. Platelet activation, expressed by a higher vWF, may not only lead to thrombotic complications in patients with atherosclerosis, but may also contribute to arterial stiffness [36].
Our study supports this thesis, but also indicates that for the early stages of arterial remodelling, endothelial dysfunction (vWF level), prothrombotic activity (TAT concentration), and presence of lupus anticoagulant may constitute significant causal factors of atherosclerosis. Interestingly, it was observed not only in patients with coronary calcifications, but also in those without calcified atherosclerosis in the coronary vessels.
Study limitations
Our study has several limitations. First, the sample size was relatively small; however, the study was adequately powered to detect differences in PWV between the study groups. Second, noninvasively measured PWV tends to overestimate PWV obtained by invasive measurements by about 20% due to the anatomical structure of individual patients and the degree of tortuosity of the aorta [37]. Third, since we evaluated a limited number of thrombotic and inflammatory biomarkers in SLE, the results of our study are hypothesis-generating. Fourth, the statistical association presented does not necessarily mean a cause-and-effect relationship.
Conclusions
In patients with systemic lupus erythematosus, PWV is related to the presence of coronary atherosclerotic lesions. In addition, arterial stiffness is higher in patients with positive lupus anticoagulant and higher levels of vWF and TAT. Lupus anticoagulant presence, endothelial dysfunction, or prothrombotic activity, manifested by higher levels of vWF and TAT, influences the early stages of arterial remodelling and atherosclerosis in SLE.