Introduction
The COVID-19 has been an ongoing health threat since the global pandemic formally started in 2020. Over time, the virulence of circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants has decreased, and improvements in therapy contributed to declining death and hospitalization rates compared to 2020–2022. Since late 2022, SARS-CoV-2 Omicron subvariants have been undergoing concurrent evolution, leading to the development of the recombinant lineage of the SARS-CoV-2 Omicron variant named XBB lineage, the dominant circulating strain at the end of 2023 in Europe [1, 2]. The XBB lineage members are not considered variants of concern; however, the risk of severe disease development remains. The primary concerns include the emergence of new variants, waning immunity, and the impact of the infection on people at high risk of developing severe disease. In 2024, COVID-19 is still expected to continue to pose a threat to specific populations. It is less likely that COVID-19 will overburden healthcare systems as it has in the past. Compared to the SARS-CoV-2 Delta variant, the SARS-CoV-2 Omicron dominance time was characterized by lower need for oxygen therapy, mechanical ventilation, and shorter hospital stay. Whereas in the early pandemic the age of hospitalized patients varied significantly, in the later phase more frequent hospitalizations concerned elderly persons with multiple comorbidities [3]. Thus, the disease could still have a negative impact on the health and lives of the most vulnerable individuals. This group includes persons of advanced age or with immunosuppression associated with underlying disease or ongoing treatment, patients with some comorbidities, pregnant individuals, and people who are unvaccinated or not up to date with COVID-19 vaccinations [4]. Despite the relatively low mortality observed during the dominance of SARS-CoV-2 Omicron variants, the mortality rate is still higher than for influenza [5]. Currently, both diseases have highly similar seasonal patterns in our climate zone, with COVID-19 still being a greater threat to people’s lives. According to epidemiologic reports, the beginning of COVID-19 season and the timeframe are less predictable than those for influenza [6].
Patients with rheumatic musculoskeletal diseases (RMDs) are at a higher risk of severe COVID-19 outcomes. The cause of this increased risk may result from a mixture of the following factors: immunosuppression from disease-associated immunoregulatory disturbances, treatment, and comorbidities [7–11]. This article aims to overview the risks and outcomes of COVID-19 in patients with RMDs. We also summarize recent findings about prophylaxis and disease management in this group of patients. Finally, we present a review of the accumulating knowledge about development of COVID-19-associated autoimmune sequelae. We believe this review will help rheumatologists stay up to date with the risks of COVID-19 for their patients and become familiarized with the possibilities to alleviate these risks. Additionally, through this publication, physicians will be able to analyze the association between autoimmune manifestations and SARS-CoV-2 infection in disease diagnosis and treatment to provide the best care possible for their patients. The presented problems and approach to SARS-CoV-2 infection management in the context of patients with RMDs result from a literature review and the authors’ opinions.
Risks associated with COVID-19
Rheumatic and musculoskeletal diseases and treatment-associated immunosuppression are significant risk factors for patients’ hospitalization and increased mortality due to COVID-19. Age over 50 remains a strong predictor of an unfavorable course of the disease, and the risk additionally increases at the age of 65 or over [4, 12]. Independently from the other factors, the risk is increased in persons with some comorbidities (e.g., lung, liver, heart, kidney diseases, diabetes, malignancies, obesity) [13–18] and certain medical conditions (e.g., pregnancy, smoking) [18, 19]. Moreover, the risk of severe COVID-19 disease course increases with the number of comorbid conditions often observed in patients with RMDs [20]. Already in 2016, the European Alliance of Associations for Rheumatology (EULAR) emphasized the role of comorbidity management in patients with chronic inflammatory rheumatic diseases. These additional health conditions require regular assessment and management in RMD patients [21, 22]. Comorbidity management remains the responsibility of rheumatologists, and it is increasingly important to evaluate the risk associated with COVID-19.
Patients with RMDs have remained at elevated risk of hospitalization due to COVID-19 since the beginning of the pandemic [23]. Even in the era of SARS-CoV-2 Omicron dominance and increased vaccination rates, RMD remains a crucial risk factor for hospitalization and death [24–26]. In the nationwide Greek cohort study conducted in the first half of 2022, patients with rheumatoid arthritis who were infected with SARS-CoV-2 had an elevated risk of hospitalization (odds ratio [OR] = 2.02, 95% CI: 1.79–2.27) and death (OR = 1.73, 95% CI: 1.36–2.20) as compared to controls [24]. Additionally, based on the data from the Danish national registers, the risk of hospitalization of patients with RMDs in the SARS-CoV-2 Omicron era decreased compared to the pre-Omicron period; however, it remained higher than in the general population [25]. The risk of death decreased substantially after the Omicron variant became the dominating strain; however, again, it was substantially higher compared to non-RMD controls [25]. Information about the COVID-19 burden in Polish patients with RMDs comes from a retrospective, non-interventional research program, SARSTer, conducted by the Polish Association of Epidemiologists and Infectiologists. According to this study, mortality due to COVID-19 was higher in patients with RMDs (42/185; 22.7%) than in adults without a rheumatic burden (1,155/8,035; 14.4%), p = 0.002 [26]. One-third of Polish patients with RMDs hospitalized with COVID-19 additionally suffered from a cardiovascular disease, as well as obesity (20.0%), diabetes (18.4%), or respiratory illness (14.6%) [26].
The COVID-19 Global Rheumatology Alliance registry, including data from 3,729 patients, reported independent risk factors associated with COVID-19-related death among adults with RMDs. Older age (> 65 years), male sex, hypertension, cardiovascular diseases, and chronic lung diseases present in patients with RMDs were associated with an increased risk of death due to COVID-19 [27]. These findings emphasize the importance of patient characteristics and comorbidities in an individual COVID-19 risk assessment.
Certain RMD treatments may also increase the risk of death from COVID-19. Among others, use of glucocorticosteroids (GCs) increases the risk of death in a dose-dependent manner. The risk ratio was doubled in patients using > 15 mg of systemic GCs daily treatment (referring to prednisone) compared to non-users (sex and age-adjusted risk ratio = 2.15, 95% CI: 1.80–2.56) [28]. In general, doses above 10 mg daily of prednisone or equivalent are considered to increase the risk of hospitalization, COVID-19-related complications, and death if taken for an extended period [29]. High doses of GCs are commonly used in the treatment of various rheumatic diseases, even without systemic inflammation, in the elderly population, and it may additionally contribute to an increased risk of death due to COVID-19 in these patients. The accumulated evidence, reviewed to inform the EULAR guidelines [29], shows that in addition to GCs, some other drugs used in RMD treatment may also increase the risk of severe COVID-19 and death, including rituximab and mycophenolate mofetil. Use of Janus kinase inhibitors (JAKi) is associated with an increased risk of hospitalization [24]. It is worth noting that some immunosuppressive therapies, including anti-IL-6R (tocilizumab) and a JAKi1 and JAKi2 (baricitinib), may have a positive impact on the outcome of severe COVID-19 [30].
In summary, the risk of COVID-19 remains increased for patients with RMDs, even during the dominance of the relatively mild SARS-CoV-2 Omicron variants (Table I). Factors increasing the susceptibility of RMD patients to COVID-19 and its consequences are primarily high comorbidity rates and the use of immunosuppressive therapies. In addition to the short-term threats of the infection, patients may also suffer from long-term COVID-19 consequences, including post-COVID-19 condition and immunologic sequelae.
Table I
Expert panel statements
| Risk of moderate-to-severe COVID-19 | During the prevalence of the SARS-CoV-2 Omicron sub-lineages, patients with rheumatic and musculoskeletal diseases require rheumatologists’ attention due to the increased risk of COVID-19 and its severe outcomes. Although the Omicron strain is associated with a reduced rate of hospitalization and mortality compared to previous variants, patients with RMDs, particularly those with high comorbidity rates and on immunosuppressive treatment, remain much more vulnerable to adverse outcomes of the infection |
| Long-term consequences of COVID-19 | Post-COVID-19 condition may concern every fifth patient with chronic rheumatic and musculoskeletal diseases. Symptoms of post-COVID-19 condition and rheumatic disease may overlap, requiring differential diagnosis The current understanding of mechanisms contributing to post-COVID-19 condition development raises concerns about the long-term consequences of autoimmunity in COVID-19 convalescents |
| COVID-19 vaccinations | All patients with rheumatic and musculoskeletal diseases should receive an updated monovalent vaccine targeting SARS-CoV-2 XBB.1.5 and future updated vaccines independently from a history of vaccination The best time to vaccinate is minimal/low disease activity and the time when immunosuppression is the lowest, i.e., adequately before or after the immunosuppressive drug, depending on its type (Table II). Patients with rheumatic and musculoskeletal diseases are vulnerable to a suboptimal immune response to the COVID-19 vaccine due to the use of immunosuppressive treatment, age, and comorbidities. Vaccination adjustments and additional doses can be required in specific clinical situations (Table II) COVID-19 vaccination may rarely result in a disease flare or autoimmunity development in a small fraction of patients; however, the benefits clearly outweigh the risks |
| COVID-19 diagnostics and treatment | Patients with rheumatic and musculoskeletal diseases are at risk of severe COVID-19 due to immunosuppression caused by the disease and its treatment, age, and comorbidities. Patients should know a detailed care plan/diagnostic-therapeutic path, which includes prompt testing at the onset of COVID-19 symptoms and rapid access to antivirals if SARS-CoV-2 infection is confirmed |
Late complications of COVID-19
The COVID-19 can lead to a range of short-term consequences, including symptoms such as fever, cough, fatigue, and in severe cases, respiratory distress, hospitalization, and death. In the long term, it may result in post-COVID-19 condition (also referred to as long COVID or post-acute sequelae of SARS-CoV-2 infection), characterized by persistent symptoms such as fatigue, breathlessness, and cognitive disturbances that can last for months after the initial infection. The ICD-10 started classifying post-COVID-19 condition as a disease in September 2020 [31]. According to the World Health Organization (WHO), post-COVID-19 condition is usually diagnosed three months after the start of COVID-19, lasts for at least two months and cannot be explained by an alternative diagnosis [32]. The knowledge about post-COVID-19 condition in patients with RMDs is limited. Post-COVID-19 symptoms often mimic manifestations of various rheumatic diseases, e.g., chronic fatigue, widespread pain, myalgia, skin rash, Raynaud’s phenomenon, cognitive impairment, or depressive mood, and have been reported in COVID-19 convalescents in the general population as well as patients with RMDs [32–35]. Reinfections additionally contributed to the increased risk of post-COVID-19 condition, which was exhibited in the general population [36].
Multiple studies have reported long-term outcomes of COVID-19 in patients with RMDs, indicating an increased burden of post-COVID-19 condition [37–39]. One prospective cohort study conducted in the Netherlands during dominance of BA.1 and BA.2 SARS-CoV-2 sublineages reported the frequency of post-COVID-19 condition in adults with inflammatory RMDs as 21%, compared to 13% in non-RMD controls. Unadjusted OR was 1.73, 95% CI: 1.04–2.87, p = 0.033, whereas confounders-adjusted OR was 1.53, 95% CI: 0.90–2.59, p = 0.12). Both groups most frequently reported fatigue and loss of fitness. Higher body mass index (OR = 1.06, 95% CI: 1.01–1.12, p = 0.015) and COVID-19 severity (OR = 3.07, 95% CI: 1.81–5.22, p < 0.0001) were associated with higher odds of post-COVID-19 condition in adults with RMDs compared to controls without RMDs [33]. Both symptoms and recovery time from post-COVID-19 condition were similar between patients with inflammatory rheumatic disease and non-RMD controls (p = 0.17) [33]. Nevertheless, it is difficult to accurately estimate rates of occurrence of post-COVID-19 condition in RMD patients, as the symptoms of post-COVID-19 condition and RMD often overlap. This may lead to overestimation, since even patients with RMDs, but without a history of COVID-19, sometimes report a considerable post-COVID-19-like symptom burden [33].
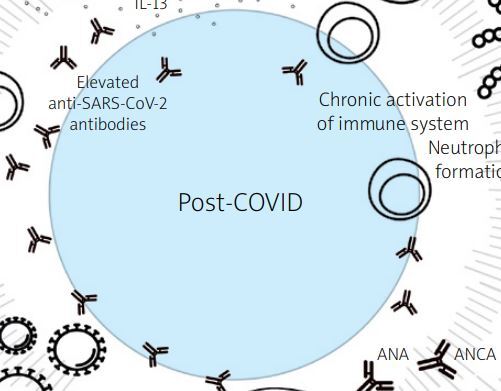
On the other hand, clinical manifestations of post-COVID-19 condition are associated with augmentation and persistence of the inflammatory response induced by infection, leading to latent and overt autoimmunity [40]. This may lead to various immune system-mediated manifestations potentially developing after COVID-19, including rheumatic disease-like symptoms [41, 42]. Among autoantibodies found in patients infected with SARS-CoV-2 were anti-nuclear, anti-neutrophil cytoplasmic, anti-phospholipid antibodies, and others (Fig. 1) [43–47].
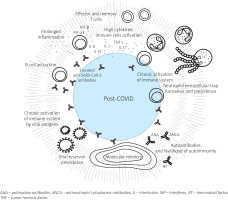
Fig. 1
Possible pathogenic mechanisms of post-COVID-19 condition. Prepared based on Fedorchenko and Zimba [47].
From the clinical perspective, distinguishing between post-COVID-19 sequelae and RMD manifestations has proven challenging and requires an extensive diagnostic strategy (Table I) [48].
Potential mechanisms of post-COVID-19 condition development
Viruses as autoimmunity triggers are relatively well characterized. Several mechanisms, such as molecular mimicry, epitope spreading, and bystander activation, can cause viral-induced autoimmunity [49]. Immune dysregulation in COVID-19 patients introduces an additional layer of complication in diagnosis, prophylaxis, and treatment. Thus, it deserves special attention from physicians treating inflammatory immune diseases, such as inflammatory rheumatic diseases.
The SARS-CoV-2 triggers a cascade of inflammatory signals, releasing various cytokines and activating the adaptive immune response. In the acute phase of the infection, an excessive increase in the levels of cytokines can cause cytokine release syndrome and/or multiple organ failure and thus may increase the risk of autoimmune conditions later on. Persistent overexpression of such proinflammatory agents is highly correlated with post-COVID-19 condition (Fig. 1) [40, 47, 50].
In the case of COVID-19, the mechanism of molecular mimicry appears to be the most important. It relies on the activation of lymphocytes specific to the pathogen and cross-reactivity with host antigens (Table I). Some degree of homology is observed between SARS-CoV-2 and human protein sequences; Dotan et al. [46] listed 34 heptapeptides shared between the pathogen and human primary protein sequences. Because of this high level of resemblance, hyperstimulated B-cells may produce antibodies against the viral components, along with autoimmune antibodies (Fig. 1) [46]. Some of them may persist after recovering from COVID-19 and eventually cause autoimmune disease. In addition, excessive neutrophil extracellular traps (NET) formation and their incomplete resolution after acute COVID-19 may cause persistent inflammation and chronic immune system activation [51, 52]. This may lead to tissue damage, immunothrombosis and thromboinflammation, and trigger autoantibody production against presented intracellular elements of NETs (Fig. 1) [47, 53]. Finally, viral persistence confirmed in autopsy studies might also serve as a chronic trigger for inflammation along with cellular activation that might result in tissue damage [53–55] (Fig. 1). The SARS-CoV-2 infection may persist longer, even after negative diagnostic tests, possibly contributing to some post-COVID-19 condition symptoms.
Inflammation, chronic immune activation, and antigen persistence, including SARS-CoV-2 differently contribute to the dynamics of COVID-19 convalescence. Increased autoantibody production may mediate autoimmune disease manifestations that could be classified as post-COVID-19 condition. Recently, an increased incidence of inflammatory arthritis was reported after COVID-19 in the Colombian population [56]. Since autoimmune diseases may manifest years after the onset of autoantibody formation [57], we will likely observe their increased incidence due to SARS-CoV-2 infections in the future. Studies are exploring the causality of the relationship between infection and new disease onset as well as post-COVID-19 condition treatment possibilities, targeting the mechanisms described above.
Rheumatic diseases following SARS-CoV-2 infection
Post-COVID-associated autoimmune dysregulation may lead to the development of various autoimmune diseases, including rheumatic diseases. In a retrospective cohort study, newly recorded autoimmune diseases were assessed in groups with positive (n = 888,463) and negative (n = 2,926,016) SARS-CoV-2 polymerase chain reaction (PCR) tests. Patients in the cohort of COVID-19 convalescents had a significantly higher risk of developing numerous autoimmune diseases, including rheumatic arthritis, ankylosing spondylitis, systemic lupus erythematosus (SLE), systemic sclerosis, mixed connective tissue disease, and polymyalgia rheumatica (PMR), as compared to non-infected patients. For these diseases, the adjusted hazard ratio (aHR) for diagnosis in the group with COVID-19 convalescents was > 2. The risk of developing the disease has increased over time since the onset of infection and is independent of the patient’s sex, race, and age. It was found that COVID-19 in an outpatient setting was associated with a higher risk of illness than in patients with a more severe course of the disease requiring hospitalization [58]. This means that anti-inflammatory drugs used during the infection may be necessary to decrease the burden of the long-term consequences of COVID-19. The increased occurrence of autoimmune diseases in patients with COVID-19 has also been documented in other studies [59–61]. Therefore, it seems important that long-term management of patients with COVID-19 should include evaluation for autoimmune disorders. Of note, some of these patients may require rheumatological care.
In summary, rheumatologists need to be aware of the potential threat of post-COVID-19 condition symptoms in patients with RMDs and the associated risk of developing new-onset autoimmune diseases in COVID-19 convalescents. Considering the high similarity of the symptoms of post-COVID-19 condition and RMDs, a thorough diagnostic process is essential to differentiate their manifestations accurately (Table I). Further research is needed to fully understand the long-term effects of COVID-19, particularly in relation to autoimmune diseases.
COVID-19 prophylaxis and management in patients with rheumatic diseases
Patients with RMDs, due to immunosuppression associated with the disease and/or its treatment, require priority COVID-19 vaccination and access to targeted medical treatments due to the more severe impact of the disease compared to the general population. The role of rheumatologists in preventing and managing COVID-19 patients with RMDs is multifaceted and critical due to the unique challenges posed by these conditions and their treatment.
COVID-19 prophylaxis
Rheumatologists play a pivotal role in the assessment of patients with RMDs, and they are responsible for guiding their patients’ COVID-19 vaccination. The European Reference Network (ERN) on Rare and Complex Connective Tissue and Musculoskeletal Diseases (ReCONNET) recommends vaccination as the most effective way to reduce the risk of severe COVID-19, hospitalization, and death [62]. The EULAR and the American College of Rheumatology (ACR) recommend against delaying vaccinations in patients with RMDs; in fact, these diseases constitute indications to vaccinate [63, 64]. Rheumatologists should address patient hesitancy motivated by fear of the vaccine’s side effects and potential worsening of RMDs, explain the pros and cons of vaccination, and clarify the patient’s potential uncertainty [65]. Vaccinations have significantly reduced morbidity and mortality due to COVID-19 and remain an essential tool to control SARS-CoV-2 infections. In the 2023/2024 season, the European Medicines Agency (EMA) and the European Center for Disease Prevention and Control (ECDC) recommend the use of updated monovalent vaccines targeting the currently dominant SARS-CoV-2 XBB.1.5 Omicron subvariant [66]. Immunocompetent persons over five years of age should receive one dose of vaccine regardless of the vaccination history, whereas for younger children the number of doses depends on their vaccination status and whether they had previously undergone COVID-19. Persons suffering from immune system disorders may require additional vaccine doses, but as of December 2023, there are no such recommendations in many countries, including Poland. In line with the latest Advisory Committee on Immunization Practices (ACIP) recommendations [67], we recommend that immunocompromised individuals receive an additional dose at least two months after the most recent dose (Table I). Patients with RMDs should be vaccinated during low activity of their disease (remission) (Table I) and, if possible, when the degree of immunosuppression is low [68]. Some immunosuppressive drugs may decrease the level of response to the vaccine [68–72], and therefore vaccination requires proper timing and/or administration of additional doses (Table II) [63]. Generally, restricting or reducing the doses of immunosuppressive therapeutics in order to undergo vaccination is not recommended, as it may increase the risk of immune system disease flare. However, it is required in some cases, e.g., when a drug is administered daily. Any decision about treatment restriction to undergo vaccination requires risk assessment by a rheumatologist. Patients at risk of a decreased response to the vaccination should receive an additional dose of XBB.1.5-adapted vaccine two months after previously receiving vaccination (Tables I, II) [67].
Table II
Recommended vaccination schedules in patients with rheumatic and musculoskeletal diseases treated with different therapies
| Drug | Negative effect on response to vaccination* | Recommended vaccine schedule |
|---|---|---|
| Rituximab | Yes [68–70, 72] | Vaccination 2–4 weeks before the next planned rituximab dose [63]. The second vaccination is advised |
| Mycophenolate mofetil | Yes [71, 72] | Vaccination 1–2 weeks after the last dose [63]. The second vaccination is advised |
| Prednisone ≥ 10 mg daily | Yes [69, 72] | The second vaccination is advised |
| Abatacept | Yes [70, 72] | Vaccination 1–2 weeks after the last dose [63]. The second vaccination is advised |
| Belimumab | Yes | Vaccination 1–2 weeks after the last dose [63]. The second vaccination is advised |
| Leflunomide | Yes [70] | Vaccination 1–2 weeks after the last dose [63]. The second vaccination is advised |
| JAK inhibitors | Yes, moderate [70] | The second vaccination is advised |
| Methotrexate | Yes, mild [69, 70] | The second vaccination is advised when the dose is > 20 mg/week [63] |
| Azathioprine | No data | The second vaccination is advised when the dose is > 2 mg/kg/day [63] |
| Cyclophosphamide | No data | Vaccination 1 week before the next planned dose [63] |
| TNF, IL-6, IL-17 inhibitors, or other biologicals modulating the immune system | No [70] | No modifications required [63] |
| Hydroxychloroquine, intravenous immunoglobulin | No | No modifications [63] |
| NSAIDs | No | No modifications [63] |
The EULAR Coronavirus Vaccine (COVAX) registry monitored outcomes of COVID-19 vaccination in 33 countries from June 2020 to November 2022. The registry enrolled 11,116 patients with inflammatory and noninflammatory RMDs. About one-third of patients (34%) experienced an adverse event after vaccination; these were mostly short-term events observed soon after the vaccination, including pain, redness or swelling at the injection site, fatigue, and fever [73]. Among the patients recruited to this study, rheumatic disease flares following vaccination occurred only in 3% of cases [73]. In another study, including 5,619 patients with various rheumatic diseases, disease worsening occurred in 4.9% [74]. Risk factors for flares here included female sex, previous adverse reactions following vaccinations, psoriatic arthritis (PsA), PMR, and SLE [74]. De Stefano et al. [75] reported flares after vaccination in 24.5% of patients with RMDs; however, these complications were most often transient and did not require a treatment change. According to the authors, exacerbations occurred most often in patients with active arthritis or those treated with medications with a short half-life [75]. Nakafero et al. [76] reported that flares were most often associated with the first COVID-19 vaccination and were less common with the subsequent doses. According to the EULAR, the frequency of flares after the COVID-19 vaccine is similar to that observed in unvaccinated patients with RMDs [64]. Thus, we conclude that the overall risk of developing an RMD flare following COVID-19 vaccination is low.
The occurrence of a new rheumatic disease due to the COVID-19 vaccination has only been documented in sporadic cases. Joudeh et al. [77] summarized reports of 127 patients (data extracted from 69 case reports or case series) with or without rheumatic diseases. Based on these reports, the authors found that de novo diagnoses occurred in patients receiving mRNA vaccines, aged > 50 years, and from 1 day to 4 weeks after vaccination [77]. Among these cases, PMR was diagnosed most frequently, with 32 cases reported [77]. Comparison of the frequency of diagnosing a new inflammatory rheumatic disease as a sequela of COVID-19 or as a side effect of the vaccination revealed an increased incidence of PMR after vaccination compared to COVID-19 convalescence (33.1% vs. 21.3%, p = 0.032) [78]. In all other cases, the frequencies of new rheumatic disease diagnoses were either similar in both groups or higher in the post-COVID-19 group [78]. According to the ACR, the risk of disease worsening, developing a flare, or a new-onset autoimmune condition after vaccination is outweighed by the expected benefits of the vaccination [63]. Thus, COVID-19 vaccines should be recommended to all patients with RMDs.
The COVID-19 vaccines significantly reduce the risk of developing severe or critical forms of the disease and help decrease COVID-19-related mortality. Receiving mRNA COVID-19 vaccines adapted to the most current SARS-CoV-2 variants reduces the risk of SARS-CoV-2 infection (HR = 0.59, 95% CI: 0.47–0.74) and severe COVID-19 (HR = 0.35, 95% CI: 0.14–0.85) among patients with RMDs using disease-modifying therapies [79]. Physicians should encourage patients with RMDs to stay up to date with COVID-19 vaccinations, recommending the XBB.1.5-targeting vaccines in the 2023/2024 season. To foster protection against infectious diseases in line with the EULAR recommendations, the COVID-19 vaccine can be co-administered with a seasonal flu vaccine, which is also recommended for most patients with RMDs [80]. Since rheumatologists play a vital role in educating their patients, they should recommend vaccinations in line with the EULAR recommendations [64, 80].
COVID-19 diagnostics
Diagnostic testing to detect SARS-CoV-2 virus infection is essential for a few reasons. Firstly, the test results may inform recommendations for the patients to self-isolate, which in turn can help limit further SARS-CoV-2 transmission. It can also provide necessary epidemiological information at a regional and country level. Additionally, and perhaps most importantly, early diagnosis protects the health and lives of patients, as it enables the initiation of antiviral treatment in patients at high risk of developing severe COVID-19. Patients with RMDs presenting symptoms consistent with COVID-19 (fever, sore throat, cough, shortness of breath, fatigue) are strongly advised to get tested, regardless of their vaccination status. Rheumatologists should serve as a source of information and education and recommend patients at risk not to postpone testing when feeling ill. Testing for the presence of SARS-CoV-2 virus is the first step on the diagnostic-therapeutic path of a COVID-19 patient (Fig. 2) [81]. Despite PCR tests remaining the gold standard, rapid antigen tests are used for convenience and speed, particularly in mass screenings or for individuals when rapid results are needed [82]. The use of at-home COVID-19 tests has become more common, providing a convenient way to check the health status, especially if a person has COVID-19-like symptoms or has been exposed to an infected person. The SARS-CoV-2 viral loads peak around the fourth day of symptoms [83]; thus, in cases of suspected SARS-CoV-2 infection, if the first test is negative, the second should be repeated 2–3 days later.
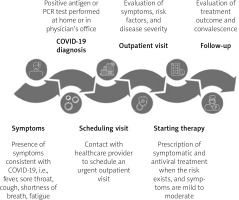
Fig. 2
Recommended diagnostic-therapeutic path for patients at high risk of severe disease and death due to COVID-19. Based on Czech et al. [81].
Rapid diagnosis is a very useful tool that can inform patients and physicians on responsible self-isolation, as well as providing valuable epidemiological information. In addition, it allows for prompt treatment initiation, which can be more effective when applied at an early stage of infection (Fig. 2) [81]. For patients at high risk of severe COVID-19, including patients with RMDs, early outpatient treatment can reduce the risk of severe COVID-19 and hospitalization or death [84].
COVID-19 antiviral treatment
COVID-19 treatment is described in detail and guided by local and international recommendations. The treatment selection depends on an individual patient’s risk of a severe COVID-19 course, which should guide optimal treatment selection: from outpatient treatment of mild disease to inpatient severe COVID-19 therapy. In addition, the severity of the disease guides the choice of therapeutics to be used, depending on the phase of SARS-CoV-2 infection [12]. Rheumatologists may intervene mainly at the early phase of COVID-19, aiming to reduce the viral load and limit the risk of progression to a more severe disease.
Effective antiviral treatment should be applied prior to COVID-19 progressing to an advanced phase of disease [62]. During the Omicron XBB.1.5 domination, directed treatment options have been limited. To prevent the development of severe, life-threatening disease requiring hospitalization, the WHO strongly recommends using the nirmatrelvir/ritonavir combination in mild to moderate COVID-19 in patients at high risk of hospitalization and also conditionally suggests use of remdesivir or molnupiravir [85]. In the European Union, molnupiravir has not received marketing authorization for the treatment of adults with COVID-19 [86]. Therefore, nirmatrelvir/ritonavir is currently the only available option for oral therapy in the European Union. The drug consists of nirmatrelvir – a cysteine (Mpro) protease inhibitor involved in the viral replication of SARS-CoV-2 – and ritonavir – a pharmacokinetic agent which slows down the metabolism of nirmatrelvir, resulting in enhanced antiviral activity of nirmatrelvir. Due to the unique mechanism of action inhibiting the effect of Mpro protease, the drug maintains effectiveness against the Omicron BA.1.1 and XBB subvariants [87]. A combination of nirmatrelvir/ritonavir is indicated for treating COVID-19 in adults who do not require supplemental oxygen and are at increased risk for progressing to severe COVID-19 [88]. It has been shown that the use of nirmatrelvir/ritonavir treatment in high-risk groups significantly reduces the risk of hospitalizations (OR = 0.45, 95% CI: 0.33–0.62, p < 0.0001), the number of emergency department visits (OR = 0.74, 95% CI: 0.63–0.87, p = 0.0002), and COVID-19-related mortality (OR = 0.15, 95% CI: 0.03–0.50, p = 0.0010), as compared to no antiviral treatment during the dominance of Omicron [89]. The effectiveness of the outpatient treatment (nirmatrelvir/ritonavir, molnupiravir, remdesivir, or combination treatment) was also confirmed in the population of patients with RMDs. The percentage of hospitalizations or deaths in the treated group was 2.1%, compared to 17.6% in the control group with no outpatient treatment (adjusted OR = 0.12, 95% CI: 0.05–0.25) [90]. Of note, early initiation of COVID-19 treatment in outpatient settings was associated with a significantly lower risk of a severe form of COVID-19 developing in patients with RMDs [90].
In line with local recommendations issued by the Polish Association of Epidemiologists and Infectiologists, therapeutics inhibiting the replication of the SARS-CoV-2 virus should be initiated in patients at high risk of severe COVID-19 within five days from the onset of symptoms [82]. In patients with documented immuno-suppression, it might be considered to start the treatment even 10 days from the onset of symptoms [82]; however, this is not supported by the product’s characteristics [88]. Standard dosing of nirmatrelvir/ritonavir consists of 300 mg nirmatrelvir (two 150 mg tablets) and 100 mg ritonavir (one 100 mg tablet), all taken together orally every 12 hours for 5 days. The dosage should be adjusted depending on the patient’s renal function. Additionally, before the implementation of nirmatrelvir/ritonavir treatment, the risk of potential drug-drug interactions should be assessed [12]. The University of Liverpool offers an open-access online tool to examine drug-drug interactions for this medicine [91], allowing physicians to evaluate the risk of interactions between COVID-19 therapies and co-medications. Nirmatrelvir/ritonavir cannot be used by pregnant and breastfeeding women or by patients with severe liver dysfunction and glomerular filtration < 30 ml/min.
Treatment with nirmatrelvir-ritonavir requires prompt action from the patient and physician to ensure that treatment is started before the acute phase of the disease. Effective antiviral treatment requires a well-established diagnostic-therapeutic pathway, which should take < 5 days; treatment should be started during this time. The first COVID-19 symptoms in patients with RMD require confirmation with a diagnostic test. Then, the patient should be examined by a physician to evaluate the symptoms, potential risks, and eligibility for an antiviral treatment. After treatment initiation, the patient should be monitored for the therapy effectiveness and safety (Fig. 2) [81]. Five-day treatment with nirmatrelvir-ritonavir reduces the risk of adverse outcomes in patients at high risk of severe infection and its consequences, including patients with immunosuppression [89] or with RMDs [90]. It is recommended that immunocompromised individuals have both adequate vaccine-based prophylaxis and a detailed care plan – a diagnostic-therapeutic path that includes prompt testing at the onset of COVID-19 symptoms and rapid access to antivirals if SARS-CoV-2 infection is detected (Table I, Fig. 2) [84].
Summary
After the acute phase of the pandemic, prophylaxis and treatment of COVID-19 in patients with RMDs is still a priority challenge since the disease and treatment-related immunosuppression and multimorbidity significantly increase the risk of hospitalization and death due to the infection. Rheumatologists play a pivotal role in recommending vaccinations and educating their patients about vigilance for symptoms and the necessity to perform a diagnostic test and initiate treatment. Here, we summarized the current state of knowledge regarding COVID-19 prophylaxis, diagnostics, treatment, and consequences in patients with RMDs. Vaccinations remain the most important tool preventing severe-course COVID-19 as well as COVID-19-related hospitalizations and death. The rheumatologists’ role in building rheumatic disease patients’ awareness and trust towards the COVID-19 vaccines is crucial. The complexity of RMDs and their treatment requires balancing vaccination timing and administering additional doses to minimize the risk of a suboptimal response. Here, rheumatologists are responsible for supporting the vaccination decision-making. Patient safety, the primary concern leading to vaccination hesitancy, comes mainly from the risk of disease flares and a theoretical risk of developing a new autoimmune condition. However, these risks are disproportional to the risks of severe COVID-19 and the potential post-COVID-19 complications, especially in this vulnerable patient population. Thus, the health benefits of the COVID-19 vaccine clearly outweigh the overall risk posed by the vaccine.
In Poland, antiviral treatment options for COVID-19 are indicated for patients at high risk of a severe course of the disease. The same indications are given by, among other organizations, the National Health Service (NHS) in the UK and the National Institutes of Health (NIH) and Food and Drug Administration (FDA) in the US.
The availability of such medical treatment is necessary to reduce the COVID-19 burden in vulnerable populations. Patients at risk should be familiar with a detailed diagnostic and treatment path in case of upper respiratory tract symptoms. This includes early diagnosis when symptoms develop, followed by rapid access to antivirals if SARS-CoV-2 infection is confirmed. Moving forward, applying the lessons learned during the pandemic to improve prophylaxis and care strategies, enhance patient support systems, and ensure better preparedness for future healthcare challenges is crucial.