Osteoporosis (OP) has been classified as a civilization disease for many years and poses an increasingly significant challenge to healthcare due to aging populations. In the European Union, around 22% of women and 7% of men over the age of 50 suffer from OP [1]. In the United States, the annual cost of treating and rehabilitating a patient after a hip fracture is around $30,000, which corresponds to approximately one-third of the cost of treating cancer [2].
Osteoporosis can be defined as a disease characterized by both a decrease in bone mineral density (BMD) and disturbances in bone microarchitecture. As a result, bones become more susceptible to mechanical injuries, leading to an increased risk of low-energy fractures, such as those resulting from falls from standing height.
According to the definition by the World Health Organization (WHO), OP is diagnosed based on dual-energy X-ray absorptiometry (DXA) examination if the T-score ≤ –2.5. The T-score, without going into details, is a measure of the standard deviation of measured BMD compared to a given population. The cutoff point of –2.5 is empirically established. Approximately 30% of postmenopausal women have been found to have such a score or lower, which accurately reflects the risk of fractures over their lifetime [3]. Unfortunately, fractures also occur in individuals with higher T-scores [4]. This is because standard DXA examinations estimate fracture risk based solely on BMD values. This means that the second factor responsible for osteoporotic fractures – disturbances in bone structure – is not taken into account.
To some extent, the aforementioned issue could be addressed by quantitative computed tomography (QCT) or radiofrequency echographic multispectrometry (REMS) examinations. Of course, both methods also have their limitations, with the greatest currently being their relatively limited availability. Furthermore, introducing a new gold standard in OP diagnosis would undoubtedly be a lengthy process. Therefore, expanding the current gold standard, namely evaluating bone microarchitecture in DXA examinations, seems to be the optimal strategy.
In DXA examinations, there has long been the possibility of assessing bone microarchitecture through the trabecular bone score (TBS). The TBS is not a direct physical measurement per se. In fact, it is a numerical method that evaluates changes in the distribution of BMD between individual pixels that make up the DXA image [5]. The more irregular their distribution, the lower is the TBS value, indicating greater disturbances in bone microarchitecture, which in turn translates to an increased risk of fractures [5, 6]. The TBS has been included in the guidelines published by the International Society of Clinical Densitometry (ISCD). However, there are still no established guidelines as strict as those for BMD assessment in DXA examinations [6]. At present, therapeutic decisions should not be based solely on TBS results.
A significant advantage of TBS is the ability to retrospectively assess examinations. Evaluating TBS requires additional software, which is increasingly being integrated into basic DXA examination analysis software. The TBS assessment itself does not require a change in the lumbar spine examination procedure. The TBS analysis is performed based on the scanned image used to assess BMD values in the L1–L4 region. Therefore, there is no additional workload required from the technician, which is undoubtedly important from the perspective of routine clinical practice.
According to our best knowledge, the largest study demonstrating the usefulness of TBS assessment is the Manitoba BMD registry study, which evaluated the results of examinations of over 45,000 patients [7]. The study fully utilized the capability for retrospective analysis. With such a large study group, the conclusions drawn can be considered significant both scientifically and clinically. Analysis of BMD, TBS, and fracture frequencies showed that in patients treated with glucocorticosteroids (GCs) and individuals with type 1 and 2 diabetes or rheumatic diseases, the TBS is a valuable tool for assessing fracture risk, much better than assessing fracture risk based solely on BMD [7]. Therefore, the TBS is an extremely valuable method for assessing fracture risk in rheumatology (Fig. 1).
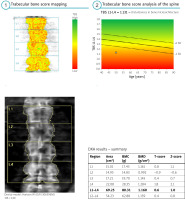
Fig. 1
The result based on BMD is normal, however, assessment based on TBS indicates significant disturbances in bone microarchitecture.
Patients with rheumatic diseases are more likely to suffer from OP than the general population, with osteoporotic fractures occurring approximately 1.5 times more often in individuals with rheumatoid arthritis than in the general population [8]. Diabetes is also a very common comorbid condition, and the high frequency of GCs use in the treatment of rheumatic diseases is well known.
The relationship between rheumatic diseases and the development of OP is complex. In this case, it is important to consider that pro-inflammatory cytokines responsible for decreased BMD are also associated with the activity of rheumatic diseases [9]. On one hand, chronic inflammatory processes lead to decreased bone density, while on the other hand, the disease process can also lead to bone remodeling [9]. An example of this is ankylosing spondylitis (AS). In AS, an active inflammatory process and the formation of syndesmophytes, which are hard bony bridges connecting adjacent vertebral bodies, are significant fracture risk factors. They artificially elevate the BMD result, thus falsifying the presence of severe osteoporosis. Syndesmophytes are associated not only with an increase in BMD values in a given vertebra but also with disruptions in bone microarchitecture [10]. As a result, fractures occur much more frequently than BMD values would suggest. In this situation, it is the TBS that better reflects the actual fracture risk.
Therapy with GCs is often used in many rheumatic diseases to reduce inflammatory activity. Paradoxically, it can also increase the risk of fractures by stimulating bone resorption, especially with chronic GCs therapy [9].
In summary, the TBS is increasingly becoming a commonly used diagnostic tool year by year, and further publications confirm its significant clinical value. It provides an important complement to fracture risk assessment based on BMD, which is particularly crucial for patients with rheumatic diseases.




