Introduction
Differential diagnosis of rheumatic diseases, especially of the systemic onset of juvenile idiopathic arthritis (sJIA), is often difficult because of the variability of clinical presentation in the absence of specific clinical and laboratory signs [1, 2].
Prior to the onset of the COVID-19 pandemic, a number of publications pointed out the diagnostic ambiguity in cases of sJIA and Kawasaki disease (KD), especially incomplete KD [3–6]. In particular, fever, rash and arthritis can be manifestations of both systemic JIA and KD [3, 7].
While many reports suggest that infections caused by the SARS-CoV-2 virus led to an overall increase in autommune diseases and rheumatic pathology [8–11], others indicate to no such increase in autoimmune diseases against the background of the COVID-19 pandemic [12]. Another challenge is presented by post-COVID or long COVID, which can manifest as arthralgia, myalgia, vasculitis, and Raynaud’s phenomenon [10].
Multisystem inflammatory syndrome in children (MIS-C) related to SARS-CoV-2 infection, which often occurs 3–4 weeks after the infection, also belongs to the group of post-COVID conditions and its symptoms are fever, skin rash, mucous membranes changes, arthralgia, myocarditis, and lesions of the coronary arteries resembling KD [7, 13].
Thus, during the COVID-19 pandemic, doctors have been facing even greater challenges in diagnosing sJIA due to its clinical features overlapping with SARS-CoV-2-related MIS-C [14, 15]. The cytokine storm of COVID-19 and hyperinflammation in MIS-C are both accompanied by fever and laboratory signs of inflammation, mimicking the systemic onset of JIA [16]. Moreover, both diseases can be accompanied by coronary artery dilatation and macrophage activation syndrome (MAS) [17], which complicate the differential diagnosis even more.
Therefore, clinicians can face challenges when diagnosing these diseases. Are these manifestations of the same disease [3, 17, 18], are they different diseases with the same clinical symptoms [14, 15], or, finally, are they different diseases that follow one another [19]? Are there specific features that would distinguish one disease from another [20]?
All these issues are currently the subject of discussion; thus, the objective of this study is to present and analyse the overlapping clinical features of sJIA and SARS-CoV-2-related MIS-C based on the analysis of a clinical case and in conjunction with a systematic review to attempt to answer the questions presented above.
Material and methods
We use a case study presentation as a basis for the discussion; it is supplemented by a search for other studies and discussions of the problem in the PubMed/Medline and Scopus databases, using the following search terms: “juvenile idiopathic arthritis” and “MIS-C”; “juvenile idiopathic arthritis” and “Kawasaki disease”. Relevant full-text articles in English published between January 2013 and December 2022 were included in this review.
Only studies with results related to clinical presentation and diagnosis were selected for the analysis. We excluded papers that mainly focused on treatment approaches.
Results
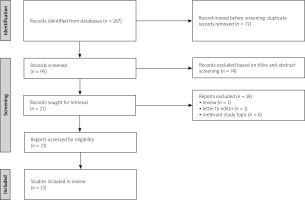
The results of the literature review are presented in a flow diagram (Fig. 1). In total, 167 publications were identified: 108 in PubMed and 59 in Scopus. Of those, 72 duplicate records were excluded. After screening titles and abstracts, 74 records were excluded as irrelevant to the study topic. Reviews and letters to the editor were also excluded. After assessing full-text articles for eligibility, 13 studies were included in the final comparative analysis.
Case description
A three-year-old girl visited a paediatric rheumatologist due to episodes of daily fever of up to 39°C for the previous 7 days. The mother reported that the child’s general condition, daily activity and appetite were satisfactory throughout the day. Her well-being was affected only during the episodes of fever. A consultation with a paediatrician three days prior the visit resulted in a diagnosis of a respiratory tract infection. Three days of antibiotic treatment did not improve general condition of the patient.
The girl was born a full-term twin by caesarean section after physiological pregnancy. The child’s physical and mental development corresponded to her age. The patient’s brother was diagnosed with autism spectrum disorder at the age of 3 years. All vaccinations were carried out according to the national schedule. Three weeks prior to the visits, the girl and her brother were suffering from acute respiratory infection, but a polymerase chain reaction (PCR) test for COVID-19 was negative.
During the physical examination there were signs of acute pharyngitis, but no evidence of other organs involvement. Complete blood count (CBC) showed anaemia, thrombocytosis, and elevated bands as well as erythrocyte sedimentation rate (ESR). Urinalysis was normal. Procalcitonin serum level was slightly increased. Ultrasound of the abdomen did not reveal hepatosplenomegaly. The patient’s electrocardiogram and echocardiography results were also normal. Lab tests showed negative immunoglobulin M (IgM) antibodies against SARS-CoV-2, and positive IgG antibodies against SARS-CoV-2 (Table I).
Table I
Clinical data, laboratory test results and treatment of the patient
Throughout the next week, the fever spiked every night. Non-steroidal anti-inflammatory drugs (NSAIDs) had limited short-term efficacy. After 7 days, CBC revealed leukocytosis (12.23 × 109/l); both thrombocyte levels and ESR were elevated D-dimer level was high (6,783 ng FEU/ml). The IgG-related antibodies against SARS-CoV-2 increased twofold in this 7-day period. Antinuclear antibodies were negative.
Systemic onset of JIA as well as other inflammatory systemic disease of connective tissue was suspected, but clinical criteria were insufficient for their diagnosis [21]. The summarized clinical date and laboratory tests results are presented in Table I.
Probable MIS-C was diagnosed considering prolonged resistant fever, laboratory indicators of inflammation, evidence of coagulopathy, and the increasing titre of anti-SARS-CoV-2 IgG. Methylprednisolone was prescribed in a dose of 1 mg/kg daily for the next 7 days, and acetylsalicylic acid 75 mg daily for one month. The patient responded dramatically on the first day of glucocorticosteroid use and upon follow-ups for the next few weeks was reporting normal body temperature and improved laboratory tests.
Six weeks after the first episode of fever, myalgia and general weakness symptoms returned. During physical examination the liver was palpated at 3 cm under the right costal border. There was no lymphadenopathy, skin rash or arthritis. Chest X-ray, electrocardiogram and electrocardiography corresponded to normal results. The anaemia persisted, white blood cell number was 9.96 × 109/l, elevated levels of C-reactive protein (CRP), procalcitonin, thrombocytes, D-dimer, fibrinogen and ESR were detected, but ferritin level was normal. Antinuclear antibodies and rheumatoid factors were negative.
In the following 2 weeks, the girl’s general condition did not improve, and her fever persisted each night. The use of ibuprofen had low antipyretic efficiency. After 2 weeks, synovitis of both knees, hips, elbows, and the second carpo-phalangeal joint of the left hand developed.
Systemic juvenile idiopathic arthritis was diagnosed. Methylprednisolone in a dose of 1 mg/kg for 28 days, methotrexate 10 mg/m2 of body surface per week, and folic acid were administered. Within the next 14 days, the synovitis symptoms resolved, and body temperature and laboratory markers of inflammation normalised. As of today, remission has been observed for one year.
Discussion
Juvenile idiopathic arthritis diagnosis, including the systemic onset of JIA, as well as the diagnosis of KD and MIS-C, is based on classification criteria. The International League of Associations for Rheumatology (ILAR) classification criteria are used for sJIA cases [21], the American Heart Association guidelines for KD [22]. The World Health Organization (WHO) and Centers for Disease Control (CDC) criteria are the ones most often used to diagnose MIS-C [23].
Some clinical criteria of sJIA, KD and MIS-C overlap [24], which creates diagnostic difficulties prompting the search for new diagnostic criteria that would distinguish one condition from another. About 1% of the patients treated for KD were ultimately diagnosed with sJIA [19].
Fever, arthritis and exanthema, elevated leukocyte and platelet counts, anaemia, hypoalbuminemia, pericardial effusion and macrophage activation syndrome (MAS) may be seen in patients with sJIA as well as in KD and MIS-C [7]. Hyperinflammation dominates in the pathophysiology of all three of these conditions, which creates a similar clinical picture, presented by fever, laboratory signs of inflammation, and involvement of various organs and systems.
To date, it has not been determined whether KD is a trigger for sJIA, since in the majority of described cases, the diagnosis of KD was made first, and later, with a repeated episode of fever refractory to treatment, it was changed to sJIA [1, 3–5]; or there was a misdiagnosis, and the initial episode of KD was actually sJIA [4]. Common triggers, susceptibility factors and immunopathogenic pathways are found in sJIA, KD and MIS-C [4]. SARS-CoV-2-related MIS-C instances have once again underscored that both viruses and genetic errors can be the causative agents of KD [13].
While a recent study [24] does not mention the overlapping clinical features, it identifies differential clinical and laboratory signs between MAS, which is a complication of sJIA, and MIS-C. In particular, ferritin, haemoglobin, lactate dehydrogenase (LDH), and fibrinogen levels were significantly different in MAS compared to MIS-C; however, the patients with MIS-C had a more severe cardiac injury.
Another study [25] showed that 21% of MIS-C patients fulfilled the 2016 classification criteria for MAS complicating sJIA [26]. Furthermore, in the patients with MIS-C, older age, atypical KD phenotype, and skin erosions were significant factors indicating the risk for MAS besides the well-known laboratory signs of MAS. In addition, the clinical course of MAS in MIS-C was milder, the prognosis better, and required treatment less aggressive than in MAS/sJIA [25].
Due to the similar clinical presentation, it is also a challenge to diagnose other COVID-19-related diseases in children with rheumatic diseases, including JIA [27]. The main characteristics of studies that analysed overlapping clinical features of sJIA and KD or MIS-C are summarised in Table II.
Table II
Studies demonstrated overlapping clinical features of juvenile idiopathic arthritis and Kawasaki disease or multisystem inflammatory syndrome in children, and a number of patients fulfilled both criteria
| Study | Country | Study design | sJIA | KD | KD/sJIA | MIS-C | MIS-C/sJIA | Distinguishing criteria |
|---|---|---|---|---|---|---|---|---|
| Go et al. [3] | Canada | Retrospective study | 112 | 1765 | 8 | – | – | Prolonged, recurrent fever and rash |
| Kanemasa et al. [1] | Japan | Nationwide survey | – | 29,084 | 18 | – | – | Refractory KD |
| Dong et al. [19] | USA | Retrospective study | – | 6,745 | 10 (0.2%) | – | – | Caucasian race, MAS, incomplete KD phenotype |
| Aydin et al. [24] | Turkey | Retrospective study | 13 + MAS | – | – | 26 | – | Ferritin, hemoglobin, LDH, and fibrinogen levels were significantly changed in MAS compared with MIS-C |
| Dogra et al. [4] | India | Case report | – | – | 1 | – | – | Refractory to i.v. Ig and infliximab treatment |
| Kumar et al. [5] | India | Case report | – | – | 1 | – | – | Refractory to i.v. Ig treatment |
| Saez-de-Ocariz et al. [7] | Mexico | Case series | – | – | 1 (+ MAS) | – | – | Not respond to i.v. Ig or complicated with MAS, present with splenomegaly and evanescent rash |
| Jagwani et al. [14] | India | Case report | – | – | – | – | 1 | Refractory to treatment |
| Waheed et al. [15] | Pakistan | Case report | – | – | – | – | 1 | Well response to antiinflammatory therapy – MIS-C |
| Han et al. [17] | Korea | Case report | – | – | 1 (+ MAS) | – | – | Chronicity of the disease |
| Ito et al. [28] | Japan | Case report | – | – | 1 | – | – | IL-18 |
| Takahara et al. [29] | Japan | Case-control study | 15 | 10 | 8 | – | – | IL-18 |
| Jiang et al. [30] | South Korea | Case report | – | – | 1 | – | – | Changes in cytokine profiles (IL-1, IL-6, IFN-γ) |
While some studies do not focus on the overlapping clinical features of sJIA and KD, they nonetheless show that the group of patients with KD had 2.02-fold greater risk of JIA compared to the general population, which may also suggest misdiagnosis of JIA at the onset of the disease [31]. Diagnosis was also complicated by the presence of arthritis in patients with KD [28].
Another study compares clinical and laboratory parameters of patients with MIS-C, KD and MAS/sJIA [32]. It shows that in the patients with MIS-C, multisystem damage of the internal organs of the cardiac, gastrointestinal, and neurological systems is more frequent compared to other conditions. Lower lymphocyte and thrombocyte levels and higher pro-BNP and ferritin levels were observed more often in MIS-C compared to KD. However, ferritin levels were the highest in MAS/sJIA [30].
The search for diagnostic markers aimed to make it possible to produce timely diagnosis of sJIA and prescribe appropriate treatment has attracted substantial attention in recent years [1, 3, 4, 29, 30, 33–35] (Table I). Some studies focus on clinical signs [1, 3–5, 7, 14, 15, 17, 19], and others on laboratory indicators [24, 29, 30]. Fever refractory to intravenous immunoglobulin (i.v. Ig) treatment was the most frequent indicator among the features of the clinical course [1, 4, 5, 7, 14, 19].
At the same time, a good response to anti-inflammatory therapy should suggest MIS-C [15]. In the case of prolonged, recurrent fever, sJIA should also be suspected [3, 17]. Among other signs, rash [3, 7], an incomplete KD phenotype [19], Caucasian race [19], splenomegaly [7], and complicated MAS [7, 19] also supported sJIA diagnosis.
Coronary aneurysms are mainly a complication of KD, and less often of MIS-C, although coronary artery dilatation can also occur in patients with sJIA, so it cannot be used for a differentiation of these diseases [3, 5, 14, 33, 36].
No specific laboratory test is available for sJIA [7]. Some researchers [33] suggest a high predictive value of ferritin levels for the differential diagnosis of sJIA and KD. However, serum interleukin-18 (IL-18) is shown to be the most useful in differentiation [20, 29, 34, 35]. High levels of S100A8/A9 and S100A12 were also helpful in distinguishing systemic JIA from KD with high sensitivity and specificity [20]. Changes in other cytokines (IL-1, IL-6, interferon-γ) in patients with overlapping signs were also studied [30].
However, there has been a reported case of incomplete KD with high levels of IL-18 and ferritin [37], which indicates the need for a complex approach to the differential diagnosis of these diseases.
Despite the fact that certain clinical and laboratory criteria have been defined for the differentiation of systemic JIA and KD or MIS-C, it is still not fully understood whether these are the extremes of the same spectrum disorder, or discrete diseases linked by the different stages of the cytokine storm.
We described a case of two episodes of the disease in a 3-year-old child, which occurred after a respiratory infection, and presented with fever, moderate changes in internal organs and systems, coagulopathy, laboratory signs of inflammation (increased CRP, ESR), and poor response to treatment with NSAIDs. The elevated anti-SARS-CoV-2 IgG titre indicated a prior episode of COVID-19.
During the first episode of fever after an acute respiratory infection, a differential diagnosis was made between systemic JIA, MIS-C, and PFAPA (periodic fever, aphthous stomatitis, pharyngitis, adenitis) syndrome. While at this stage there were not enough criteria for the diagnosis of the systemic onset of JIA [21], the small number of episodes of fever and short duration of the disease ruled out PFAPA-syndrome [38].
A diagnosis of MIS-C according to the WHO criteria [23] was supported by fever for more than 3 days; elevated markers of inflammation (ESR, CRP, and procalcitonin); no other microbial cause of inflammation (ineffective antibiotic therapy); and evidence of SARS-CoV-2 infection (positive anti-SARS-CoV-2 IgG). However, clinical symptoms were scarce. Among the clinical manifestations, we observed only the presence of mucosal changes (pharyngitis), myalgia, weakness and evidence of coagulopathy (elevated D-dimer and fibrinogen).
Therefore, probable MIS-C was diagnosed, which was supported by studies indicating that MIS-C should be considered in all children with unexplained fevers during the ongoing COVID-19 pandemic [39]. The child responded well to the glucocorticosteroid therapy, with positive dynamics of clinical and laboratory indicators. However, 6 weeks later, the episode of fever recurred with the subsequent addition of hepatomegaly and polyarthritis, which made it possible to diagnose systemic onset of JIA.
A noteworthy characteristic of this case is normal ferritin levels during both the first and second episodes of fever, which complicated the differential diagnosis. As mentioned above, elevated ferritin is described as an effective marker of differential diagnosis [24, 33].
We suggest that in our patient her anaemia could have caused normal ferritin levels. Thus, this clinical case demonstrates that ferritin may not always be an effective marker for differential diagnosis. In addition, we did not observe a rash, which is a common symptom in both MIS-C and sJIA.
A specific fever pattern with a spike occurring once per day, mainly at night, with temperature returning back to normal and relatively satisfactory well-being of the child during the day supported sJIA; however, the increasing titre of anti-SARS-CoV-2 IgG and signs of coagulopathy pointed to MIS-C [23, 40].
In general, analysis of the present case shows that prolonged, recurrent fever with a specific pattern supports sJIA diagnosis. Laboratory indicators help in diagnosis, but do not always play a decisive role.
Thus, shared pathogenetic mechanisms and similar clinical and laboratory features complicate differential diagnosis of MIS-C and sJIA in the era of COVID-19. We should also remember that not all febrile children with elevated inflammatory markers and positive SARS-CoV-2 IgG are unquestionably patients with MIS-C [14].
Although the classification criteria are crucial, in clinical practice it is difficult in some cases to diagnose the diseases according to these criteria and the clinician’s expertise/expert opinion may also be important.
Conclusions
Fever and laboratory markers of inflammation are overlapping features of sJIA and SARS-CoV-2-related MIS-C, which complicates diagnosis in the era of COVID-19 pandemic.
In children with symptoms of prolonged, spiking, unexplained, recurrent fever with a specific pattern, sJIA should be considered in the differential diagnosis.