Introduction
Systemic sclerosis (SSc) is characterized by skin and organ fibrosis (due to the activation of fibroblasts and excessive collagen production) and microvascular disease. The presence of another rheumatological condition in patients with SSc is not uncommon.
In a recent study [1], 6.4% of SSc patients studied were identified as having an overlap syndrome. The most frequent SSc overlap syndromes reported were myositis (42.8%) and rheumatoid arthritis (RA) in 32% [2]. It is known that there is a higher incidence of RA in SSc patients than in the general population [3, 4]. Therefore, an active search for overlap syndromes should be performed in all patients.
Scherlinger et al. [1], reported that overlap and non-overlap SSc presented similar characteristics, including comparable incidences of organ manifestation or severity of systemic involvement. However, they found differences in terms of treatment and prognosis [1].
The treatment is still a challenge because there is no available drug with a clear beneficial effect on the natural history of the disease. The early diagnosis and treatment of RA predict better outcomes and raise the probability to achieve remission/low disease activity [5]. This is important since most overlap syndrome patients have an erosive disease and few of them achieve remission [1].
We here report a case of a patient with SSc-RA overlap with severe pulmonary and cardiac manifestations and perform a review of the cases reported in the literature to gather the data available about this issue.
Material and methods
Case-based review
The literature search was performed in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) for articles published up to 28th March 2022. We selected published articles in English, Portuguese, Spanish or French. To identify the relevant studies, Medical Subject Headings (MeSH) and key words related to “Systemic Sclerosis” and “Rheumatoid Arthritis” were used.
The search strategies used to identify relevant studies are given in Table I. Patients who were diagnosed with SSc-RA overlap were included. The following study designs were allowed: cohort studies, case series, and case reports to gather information about the clinical, biological, and demographic features of these patients. Anti-ribonucleoprotein (anti-RNP) positive patients were excluded.
Table I
Search strategy
Titles, abstracts, and full texts were all screened for inclusion. Data was collected from each chart in a case sheet and included: demographic features of the patients, full details about their diagnosis and symptoms, organ involvement, blood test results, and medications prescribed.
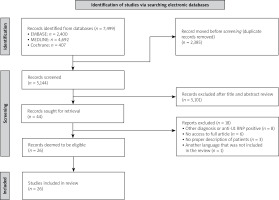
A workup of the identified records according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) scheme was conducted (Fig. 1). EndNote 20 was used to manage the references obtained from the search results of each of the databases.
Our search retrieved 7,499 articles (407 in Cochrane, 2,400 in EMBASE, and 4,692 in MEDLINE). Of these, we excluded duplicates (2,385) within and across databases and the remaining 5,114 articles were assessed. After a review of the title and abstract, 44 articles were retrieved for full-text evaluation, of which we included 26 articles in the case-based review, as shown in Figure 1. Articles included in the systemic lupus erythematosus are shown in Supplementary Table [17–41]. The excluded articles and the reasons for exclusion are shown in Table II [42–58].
Table II
Articles excluded and reason(s) for exclusion
| Reference | Exclusion criteria |
|---|---|
| Chimenti et al. [42] | Other diagnosis (localized scleroderma) |
| Hernández-Beriain et al. [43] | Without access to the article |
| Hrycaj et al. [44] | Without access to the article |
| Iwata et al. [45] | Anti-U1-RNP positive |
| Losada et al. [46] | Without access to the article |
| Nanke et al. [47] | Anti-U1-RNP positive |
| Noda et al. [48] | Other diagnosis (localized scleroderma) |
| Norbis et al. [49] | Without access to the article |
| Ohtsuka et al. [50] | Other diagnosis |
| Pakozdi et al. [2] | Not possible to characterize properly the patients |
| Radulescu et al. [51] | Other diagnosis |
| Schüller Pérez et al. [52] | Other language |
| Sherman et al. [53] | Without access to the article |
| Tsuji et al. [54] | Not possible to characterize properly the patients |
| Tuffanelli et al. [55] | Without access to the article |
| Turkcapar et al. [56] | Anti-U1-RNP positive |
| Wielosz et al. [57] | Not possible to characterize properly the patients |
| Zuckner et al. [58] | Other diagnosis |
The included patients’ characteristics are presented in Table II. Sixty-three patients were analyzed in this review, 51 females (80.95%) with a mean age of 45.03 ±14.84 years at the time of the first diagnosis. More than half of studied patients (n = 36, 57.14%) were diagnosed first with SSc, 15 (23.81%) were diagnosed with RA first and 7 had both diagnoses at the same time.
In 5 cases it was not reported which was diagnosed first. Thirty-six patients (57.14%) were diagnosed with limited cutaneous SSc (lcSSc) and 24 patients (38.10%) were diagnosed with diffuse cutaneous SSc (dcSSc); in 1 patient the cutaneous involvement was not reported, and in the other was non-existent (SSc sine scleroderma).
Regarding organ involvement in SSc, 61 patients (96.83%) presented cutaneous involvement, 54 (85.71%) presented vascular involvement, 45 (71.43%) presented pulmonary involvement, 24 (38.10%) gastrointestinal involvement, 11 (17.46%) cardiac involvement, and 4 (6.34%) presented renal involvement.
Almost all the included patients presented arthritis, which can be a manifestation of both diseases, and 41 patients (65.08%) were described as presenting erosive arthritis. The time between the first and the second diagnosis was 9.99 ±11.86 years.
Regarding autoantibodies related to SSc, a few reports detailed these, but 24 (38.10%) were anti-Scl-70 positive and 11 (17.46%) were anti-centromere antibody positive. Concerning RA-related autoantibodies, 56 (88.89%) presented positive rheumatoid factor (RF), and 29 (46.03%) presented positive anti-citrullinated protein antibody.
Concerning the treatment, the most prescribed medication were glucocorticosteroids in 20 patients, and methotrexate in 14 patients. Non-steroidal anti-inflammatory drugs were used in 9 patients, 7 patients used sulfasalazine, 5 patients used tocilizumab or hydroxychloroquine, 3 patients used etanercept, 2 patients were prescribed with rituximab (RTX), infliximab, baricitinib, tacrolimus or azathioprine (AZT) and 1 with abatacept, adalimumab or leflunomide. Ten patients used D-penicillamine and 7 patients were prescribed with gold salts however, these reports are older and currently, rheumatologists do not use these medications.
Thirteen patients reported associated diseases, as follows: 3 patients presented Sjögren’s syndrome (1 of them presented amyloidosis), 2 primary biliary cirrhosis, 1 systemic lupus erythematosus, 1 anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, 1 polymyositis, 1 Histoplasma capsulatum tenosynovitis, 1 bilateral vocal fold immobility, 1 cardiac AA amyloidosis, 1 Sweet syndrome and 1 presented hypothyroidism.
Case description
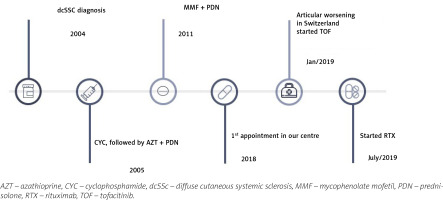
A chart review of the present patient was performed. The patient consented to the use of his clinical data and agreed with the publication of the article. A 56-year-old man, previously followed in another rheumatology department by diffuse SSc (dSSc) with anti-topoisomerase I (anti-Scl-70) antibody positivity with multisystemic involvement, was referred to our department. Figure 2 shows a global view of the patient’s disease.
The patient presented a Raynaud’s phenomenon for 14 years, which prompted the installation of a cervical medullar stimulator. Pitting scars and digital ulcers complicated the course and he started iloprost. Other signs presented were telangiectasias, microstomia, and scleroderma. Later, he was diagnosed with interstitial lung disease (ILD) and started cyclophosphamide (CYC), completing 12 cycles (cumulative dose 9 g/m2).
Then initiated AZT, which was switched to mycophenolate mofetil (MMF) due to pulmonary worsening with inefficacy of the treatment. One decade later, the patient presented worsening of the articular involvement and was started on prednisolone (10 mg/day).
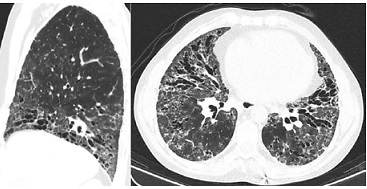
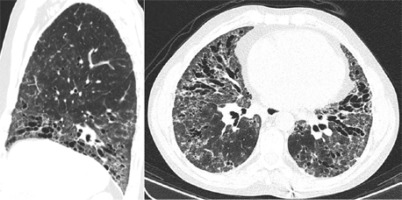
At our first appointment, the patient presented with dyspnoea for small efforts, puffy hands, polyarthritis, and skin thickening (high results in modified Rodnan total skin thickness score – 22). An endoscopy was performed, since the patient had dysphagia and showed congestive gastropathy. Spirometry showed a low diffusion capacity for carbon monoxide (26.5%). High-resolution computed tomography (HRCT) identified ground-glass opacities, and abundant bronchiectasis of the lower lung and mediastinal, subcarinal, and pre-tracheal adenopathies The HRCT scan was more typical of usual interstitial pneumonia (UIP) pattern with characteristic honeycomb (Fig. 3).
Fig. 3
High-resolution computed tomography showing ground-glass opacities, and abundant bronchiectasis of the lower lung and mediastinal, subcarinal, and pre-tracheal adenopathies.
A 6-minute walk test was performed and he walked 400 meters in 6 minutes; a desaturation in the first minute to 85% was verified. An echocardiogram was performed, and a systolic pulmonary artery pressure (SPAP) of 40 mmHg was described. The patient was referred to a further pulmonary hypertension diagnostic with a right heart catheterization (SPAP of 29 mmHg was described) with a pulmonary capillary wedge pressure 14 mmHg.
The diagnosis of pulmonary arterial hypertension was established and treatment with sildenafil 150 mg/day p.o. and bosentan 250 mg/day was initiated and more recently bosentan was switched to macitentan 10 mg/day with symptoms improvement.
One year later, the patient travelled to Switzerland on holiday and presented a worsening of his arthritis. Among other tests, X-ray of the hands was performed and on radiographs erosions were found. In blood tests anti-citrullinated protein antibodies at high levels (> 340 U/ml) and positive RF (50 IU/ml) were present so the diagnosis of RA also was made and treatment with tofacitinib (10 mg/day) was started.
Six months later, the patient presented high RA activity (Disease Activity Score with 28-joint counts [DAS28] 6.34). Therefore, it was decided to start RTX taking into consideration the pulmonary involvement in a course of SSc. After one month, the patient presented a reduction in RA activity (DAS28 5.63), and 2 months later presented a great improvement with controlled disease activity (DAS28 1.25). Also, a slight improvement in SSc symptoms and stabilization of pulmonary involvement were noted, as shown in Table III.
Table III
Functional respiratory tests through the following up period in our hospital
Discussion
Our aim with this case-based review was to describe the characteristics of patients with SSc-RA overlap reported in the literature. The cases predominantly concern women (80.95%) and in the majority of the cases, the first diagnosis was SSc. The time between diagnoses was 9.99 ±11.86 years. Early diagnosis of RA is often difficult in patients with SSc because arthritis may be present in both [6].
In our patient, RA was diagnosed almost 14 years after the SSc diagnosis. As arthritis is a clinical feature of both diseases, initially as the majority of the patients have cutaneous manifestations, the arthritis is considered an SSc feature and doctors tend not to search for other explanations.
However, the main characteristic that can differentiate both, is that RA is associated with erosive arthritis. In our case-based review, patients with overlap presented erosions in 65.08% of cases. However, some reports did not describe erosive arthritis, which could open the possibility for a reporting bias.
There is a higher prevalence of overlap in lcSSc (57.14%). Similarly, in a study by Horimoto and da Costa [7], 50% of the patients with SSc-RA overlap had lcSSc. Since erosive arthritis with positive RF and scleroderma-like skin changes can be seen in mixed connective tissue disease (MCTD), we excluded all cases with anti-U1-RNP autoantibody positive.
However, autoantibodies were not described in a large proportion of the reported cases, which can also be considered a reporting bias. Regarding the SSc organ manifestations, the most frequently reported were cutaneous (96.83%), vascular (85.71%), pulmonary (71.43%) and gastrointestinal (38.10%) involvement.
Pulmonary manifestations are the leading cause of morbidity and mortality [8]. Interstitial lung disease occurs in up to 80% of SSc patients, making ILD more prevalent in SSc than in any other connective tissue disease [6]. The main risk factors for lung fibrosis are male sex, diffuse cutaneous SSc subtype, presence of anti-Scl-70 antibodies, forced vital capacity of < 70%, and extent of fibrosis at baseline [9].
The most common pattern of interstitial pneumonia in SSc is nonspecific interstitial pneumonia (NSIP) with a fibrosing pattern. The histological features in RA patients can be UIP or NSIP, with UIP being more common [10–12].
Also, inflammatory small airway disease might be a secondary finding in RA [6]. In this case, our patient presented with pulmonary involvement of both diseases, fibrosis and bronchiectasis. In systemic sclerosis RA overlap, other manifestations besides arthritis can overlap, as our case report showed with an overlap of pulmonary manifestations of both diseases.
The optimum treatment of RA-ILD and SSc-ILD remains to be established. Immunosuppressive drugs such as CYC and MMF are still the most widely used [13]. Both showed modest improvements in our patient and a worsening of arthritis justified the introduction of tofacitinib.
A few studies have found evidence that tofacitinib could have beneficial effects in scleroderma and vasculopathy [14, 15]. However, our patient maintained high activity disease and respiratory complaints. For this reason, we switched to RTX.
Currently, there are case reports and a few open-labelled, uncontrolled studies on RTX in the treatment of SSc. Although there are inconsistencies due to different study designs, there is evidence of an antifibrotic effect of RTX. In all studies, an improvement in lung function and dyspnoea functional class was verified [16].
Study limitations
One limitation of this case-based review is that most of the cases do not report the SSc profile of autoantibodies, and some of these cases can be misdiagnosed (for example an MCTD).
Another limitation is that a major part of the articles are not recent, which explains the reported outdated treatment and cannot be extrapolated for the current times.
Conclusions
Revision of the literature to gather available information about the cases of overlapping of SSc and RA was presented with clinical picture of the severe case of SSc-RA overlap. Screening for an associated disease should be encouraged with a proper profile of autoantibodies since the overlap with SSc may affect prognosis and treatment.
This review might be of interest, since we collected clinical and serological data regarding the overlap, and we hope that raises awareness of clinicians to actively search for associated diseases, as this could lead to an early diagnosis of the overlap and to prompt treatment for articular symptoms of these patients (Supplementary Table online).