Introduction
Polyarteritis nodosa (PAN) is a necrotizing vasculitis characterized by inflammation of medium-sized arteries, causing microaneurysm, stenosis, and thrombosis, therefore leading to ischemia or bleeding of the supplied tissues [1]. The main manifestations of this vasculitis are weight loss, fever, hypertension, peripheral neuropathy, skin involvement, gastrointestinal disease and renal involvement [1].
Peripheral neuropathy is one of the most frequent and earliest symptoms, affecting 50–75% of patients with PAN and typically results from focal or multifocal, axonal ischemic neuropathy caused by arteriolar occlusion of the vasa nervorum, usually of epineurial arteries [2–4].
Central nervous system involvement affects only 2–10% of patients with PAN, usually arises late during the disease course and symptoms are highly variable and depend on the affected territory [3, 5]. Cranial nerve involvement in PAN is underrecognized and has rarely been reported. Cranial nerve palsy, most often involving the oculomotor (III), trochlear (IV), abducent (VI), facial (VII), and vestibulocochlear (VIII) nerves, affects less than 2% of patients with PAN [3].
The aim of this review is to analyze the reporting in the literature of the problem of oculomotor nerve palsy in the course of polyarteritis nodosa.
Material and methods
Systematic literature review
A review of the literature was done by searching PubMed, using the terms “polyarteritis nodosa”, “nerve”, “oculomotor”, “cranial nerve” and “cranial neuropathy”. All articles resulting from these searches were screened by the language (English), title and abstract and the eligible ones were kept for full-text review. References were additionally searched and reviewed.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for Individual Patient Data systematic reviews (PRISMA-IPD) was used (Fig. 1).
Fig. 1
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of searching methods.
This search showed only 16 reported cases of PAN with cranial neuropathy (Fig. 1). Each case is described in Table I [6–21]. In 10 of the 16 patients, cranial neuropathy was the initial manifestation, as in our case. The most frequent cranial nerve involved was the optic nerve (in 10 of the 16 cases, 62.5%), followed by the oculomotor nerve, involved in 3 cases [8, 11, 21]. No involvement of olfactory, glossopharyngeal, vagus, accessory and hypoglossal nerves was reported, according to our literature review. The majority of these patients were treated with corticosteroids and cyclophosphamide and presented variable outcomes, as described in Table I.
Table I
Demographic features, presenting symptoms, treatment and outcome of reported cases of polyarteritis nodosa with cranial nerve involvement.
| Authors and article | Age [years] | Sex | Initial manifestation | Presenting symptoms | Cranial nerve involved | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Borruat et al. [6] | 51 | F | Yes | Diplopia, myalgia, fever, hypoesthesia of the left foot. Later, sudden bilateral visual loss | II and IV | PDN, CYC | No improvement of visual deficits |
| Emad et al. [7] | 23 | F | No | Sudden unilateral visual loss, fever, weight loss and anorexia | II | MP, CYC | Partial recovery of visual acuity |
| Engel et al. [8] | 5 | M | No | Fever, right hemiparesis, pupil-sparing third cranial nerve palsy, hypertension and intracranial hematoma | III | Supportive treatment for intracranial hemorrhage | Death |
| Graf et al. [9] | 26 | F | Yes | Decreased visual acuity, hypertension and mononeuritis multiplex in the right upper and lower extremities | II | PDN, CYC | Partial recovery of visual acuity |
| Hsu et al. [10] | 70 | F | Yes | Fatigue, weight loss, decreased visual acuity and hypoesthesia of the right foot and right hand | II | PDN, CYC | Partial recovery of visual acuity |
| Hutchinson [11] | 36 | M | Yes | Posterior ischemic optic neuropathy, right III and VI nerve palsies | II, III, VI | ACTH 80 units/day | Resolution of extraocular muscle palsies |
| Kostina-O’Neil et al. [12] | 39 | M | Yes | Decreased visual acuity, subdural hematoma, weight loss and acute epididymitis | II | MP, CYC, interferon, ribavirin | Partial recovery of visual acuity |
| Lake-Bakaar et al. [13] | 63 | F | Yes | Tinnitus and bilateral sensorineural deafness, muscle weakness in extremities and arthralgias | VIII | PDN, AZT | Resolution of auditory involvement |
| Long et al. [14] | 23 | F | Yes | Sudden bilateral visual loss and weakness of left foot dorsiflexion | II | PDN, CYC | Resolution of visual deficit |
| Ouellette et al. [15] | 11 | F | No | Fever, rash, ataxia, left hemiparesis, cranial nerve palsies | V, VI, VII (questionable IX and X) | PDN, heparin, CYC | Partial recovery with hemiparesis |
| Sedwick et al. [16] | 79 | M | Yes | Diplopia, deficits of abduction, fever, nonspecific weakness and arthralgias | VI | PDN | Complete recovery |
| Sener et al. [17] | 23 | M | Yes | Sudden unilateral visual loss, weakness on right foot, hypoesthesia in extremities and hypertension | II | MP, CYC, HOT | Small improvement of visual deficits |
| Ueno et al. [18] | 34 | F | No | Sudden unilateral visual loss, muscle weakness and dysesthesia in lower limbs | II | MP, PDN | Complete recovery |
| Vazquez-Romo et al. [19] | 41 | M | Yes | Decreased visual acuity, testicular pain and cutaneous nodules | II | MP, CYC | Residual defect of visual fields |
| Yuminaga et al. [20] | 53 | M | No | Bilateral testicular swelling, fatigue, myalgias, fever and facial nerve palsy | VII | MP, CYC | Complete recovery |
| Wahezi et al. [21] | 1 | F | No | Blurry vision, diplopia, left-sided ptosis and inability to adduct left eye | III | MP, CYC | Complete recovery |
| Current case | 61 | M | Yes | Weight loss, anorexia, numbness on the lower limbs, diplopia, ptosis and oculomotricity changes on the left eye | III | MP, AZT | Complete recovery |
Case description
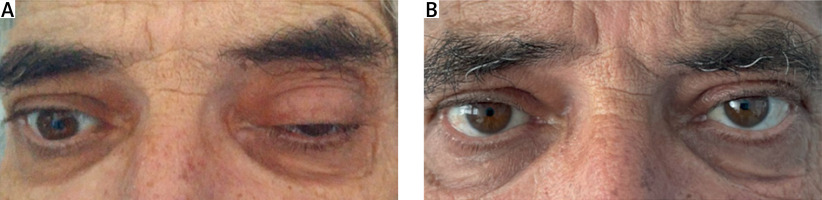
We report a case of a 61-year-old man, smoker, with arterial hypertension, who was admitted to our hospital with a 4-month history of constitutional symptoms (16% body weight loss and anorexia) and numbness of the plantar surface of the feet, and since the day before, with diplopia, ptosis and sudden changes in oculomotricity in the left eye (Fig. 2). No family history of rheumatic or neurologic diseases was reported.
The patient’s vital signs were normal, except for blood pressure which was high. On physical examination, he looked underweight. Neurological evaluation revealed weakness of ankle dorsiflexion (grade 4/5 according to the Medical Research Council scale), with normal plantar flexion, “glove and stocking” dysesthesias distal to the knees, reduced pallesthesia distal to the iliac crest, complete ptosis and exotropia of the left eye and anisocoria greater in bright light. Slit lamp and fundus examination of both eyes was normal, with a normal sized optic disc. Visual acuity was also normal.
Beside elevated inflammatory markers (erythrocyte sedimentation rate – ESR 51 mm/h, C-reactive protein – CRP 20.2 mg/l), laboratory workup was normal. Autoimmune workup, including antinuclear antibodies (ANA), rheumatoid factor (RF), anti-cyclic citrullinated peptide (ACPA) antibodies, antineutrophil cytoplasmic antibodies (ANCA), antibodies against extractable nuclear antigen (ENA), antineuronal antibodies and cryoglobulins, was negative and complement levels were normal. Angiotensin converting enzyme (ACE) and ceruloplasmin levels were normal.
Negative hepatitis B surface antigen (HbsAg), positive hepatitis B surface antibody (anti-HBs) and positive hepatitis B core antibody (anti-HBc) were found, consistent with a past infection. Hepatitis B surface antigen (HbsAg) was negative. Serological testing for Epstein-Barr virus, cytomegalovirus, herpes simplex viruses types 1 and 2, parvovirus b12 and human immunodeficiency virus was negative.
Electromyography (EMG) of the limbs showed a severe, acute and symmetric distal axonal sensorimotor polyneuropathy. To look for underlying malignancy, computed tomography (CT) of chest, abdomen and pelvis and endoscopic evaluation were performed and no evidence of malignancy was found. Chest CT scan revealed a cluster of small nodules in the superior lobe, associated with tree-in-bud lesions and no lymphadenopathy was found. Sputum examination did not reveal acid-fast bacilli and cultures were negative. Bronchoalveolar lavage mycobacterial culture and polymerase chain reaction for detection of Mycobacterium tuberculosis were negative.
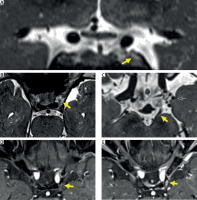
The first brain magnetic resonance imaging (MRI) was unremarkable. A second study was performed and the MRI angiography revealed a small aneurysm of the left posterior communicating artery (Fig. 3), without compression of the oculomotor nerve pathway (Fig. 4 C). Additionally, heavily T2-weighted sequences showed thickening and hyperintensity of the left oculomotor nerve (Fig. 4 A, B), with avid enhancement after gadolinium administration, more conspicuous on fat-saturated T1-weighted images (Fig. 4 D, E).
Fig 3
3D TOF MR angiography revealed a small aneurysm of the left posterior communicating artery (arrow).
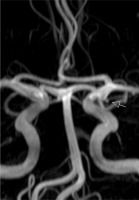
Fig. 4
Brain MRI: coronal (A) and axial (B) T2-weighted images showing thickening and hyperintensity of the left oculomotor nerve. On another heavily T2 weighted axial plane (C), it’s possible to identify the aneurism of the left posterior communicating artery (black arrow), without anatomical relation with the oculomotor nerve pathway (yellow arrow). Additionally, it also exhibited avid enhancement on postgadolinium fat-saturated T1 weighted images (D, E).
Polyarteritis nodosa with cranial and peripheral neuropathies was diagnosed. Intravenous methylprednisolone pulse (1 g daily for 3 days), followed by 0.5 mg/kg daily of oral prednisolone and azathioprine (AZT) (2 mg/kg daily), was started. At 3 months’ follow-up, a huge improvement was observed with resolution of the diplopia and oculomotricity changes, and improvement of the numbness (Fig. 2 B). Also, inflammatory markers returned to normal and, EMG showed improvement of the polyneuropathy. Prednisolone was slowly tapered and 2 years later the patient remains in remission on AZT.
Discussion
Cranial nerve involvement in PAN is a rare manifestation, being less frequent than the central nervous system involvement and peripheral neuropathy. According to our literature review, the most frequent cranial nerves involved in PAN are the optic (II), oculomotor (III) and abducent (IV) [6–21]. We did not find any case report with involvement of olfactory, glossopharyngeal, vagus, accessory or hypoglossal nerves in patients with PAN.
The present case of cranial involvement, more specifically oculomotor nerve involvement, was an onset manifestation of PAN. This is an unusual manifestation that can lead to debilitating consequences in the absence of the appropriate treatment, such as diplopia, pupil mydriasis, and/or upper eyelid ptosis.
The most frequent causes of acquired oculomotor nerve palsy are microvascular, followed by trauma, malignancy, post-neurosurgery and aneurysm [22]. Despite the existence of the posterior communicating artery aneurysm, no compression of the oculomotor nerve was observed in our case. Furthermore, the thickening of the left oculomotor nerve with enhancement on post-gadolinium sequences and a full recovery with immunosuppression therapy supports the diagnosis of cranial nerve involvement secondary to PAN.
The other suggestive manifestations of a vasculitic etiology helped to establish the diagnosis. Exclusion of other potential causes is also important. Hepatitis B serology should always be requested, as this infection can be associated with the development of PAN and in such cases the treatment is based on antiviral therapy (e.g. entecavir, telbivudine, tenofovir) with evaluation of the activity(replication) of the virus.
Treatment of cranial involvement in PAN without hepatitis B infection included glucocorticosteroids, as first-line treatment in monotherapy to rapidly control inflammation. Iimmunosuppressive agents, such as cyclophosphamide or AZT with combination with GCs may be a second line treatment [6–21]. The described patient responded very well to intravenous methylprednisolone pulse therapy, followed by oral prednisolone and AZT, remaining in sustained remission with AZT in monotherapy. However, other patients with PAN needed more aggressive treatment, with cyclophosphamide, and not all of them had a full recovery.
In some cases of severe PAN the use of rituximab (anti-CD20) monoclonal antibody was reported. Plasma exchange has narrow indications for some situations of PAN associated with HBV infection [23].
Conclusions
Polyarteritis nodosa should be considered in the differential diagnosis of a patient with cranial neuropathy, especially in the presence of concomitant peripheral neuropathy, constitutional symptoms, skin manifestations, hypertension, hepatitis B virus infection and other suggestive vasculitic manifestations.
Cranial neuropathy can occur early in the course of PAN and precede other organ disease, which makes diagnosis more difficult. Clinician awareness of the spectrum of neurologic disease in vasculitis is required to reduce diagnostic delay, to promote prompt diagnosis and treatment, and to provide a better prognosis.