Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disorder in children, lasting > 6 weeks and beginning before 16 years of age [1]. Juvenile idiopathic arthritis is also considered a possible cause of short- and long-term disability in children [1]. Juvenile idiopathic arthritis has replaced the former name juvenile rheumatoid arthritis (JRA) and comprises a heterogeneous group of conditions with an estimated prevalence of 1–4 per 1,000 [1].
There is no clear treatment for JIA and its etiology is also unknown. Treatment of JIA is critical because untreated JIA can lead to permanent disability of the affected individual due to the complications of this disorder. Complications of JIA include joint destruction, growth failure, and blindness (in a group of patients).
The aim of treatment is to reduce the frequency of relapse, maintain proper joint function, and prevent progressive damage [2]. In patients suffering from the systemic form of the disorder the importance of appropriate treatment is even more pronounced. If left untreated, systemic JIA leads to multiorgan failure [3, 4].
Contrary to the earlier assumption that JIA activity is suppressed upon reaching adulthood, recent studies have shown that in some patients, JIA can persist into adulthood with periods of relapse and remission [5]. As a result, many JIA patients require endless therapy, which places a psychological and economic burden on both families and society.
The therapeutic protocol for JIA has changed significantly over time. Significant improvements have been made in the therapeutic protocol and medications for JIA over the past decade. Currently, drug therapy for JIA includes non-steroidal anti-inflammatory drugs (NSAIDs), including naproxen and ibuprofen, in addition to disease-modifying antirheumatic drugs (DMARDs) and more recently biologic agents [6].
Among DMARDs, methotrexate (MTX) is the first line drug for JIA. Prescribed at a dose of MTX 6 mg/m2 weekly it is effective in reducing joint related signs and symptoms in the majority of patients [7, 8]. However, it appears that many patients do not respond adequately even to high doses of MTX, and many patients develop tolerance or are unable to continue therapy because of side-effects.
Some studies have suggested the use of other drugs in addition to or instead of MTX. One of these drugs, which is also a DMARD, is leflunomide (LFN) [9, 10]. Leflunomide is converted to its active form in the human body and competitively inhibits dihydroorotate dehydrogenase (DHODH) to decrease pyrimidine production.
Although the exact mechanism of action of LFN in rheumatic disorders is not yet known, it has been used successfully in adult rheumatoid arthritis (RA) with equivalent efficacy to sulfasalazine [11]. To the best of our knowledge, few studies have investigated the effect of LFN in JIA patients [9, 10, 12–14].
Some of these studies were retrospective, and a few studies assessed the effects of concomitant administration of LFN and MTX in JIA patients. Two widely used clinical assessment tools to evaluate treatment response to treatment in JIA are the American College of Rheumatology Pediatric criteria (ACRPed) and Childhood Health Assessment Questionnaire (CHAQ38) scores. Both instruments have also been validated and found to be suitable for clinical trials [15, 16].
The primary ACRPed was based on a 30% improvement in three of six criteria, which was referred to as the 30%. After the widespread use of ACRPed 30%, 50%, 70%, and 90% were also introduced [15, 17].
The aim of this study was to compare the efficacy of adding LFN to the conventional treatment regimen with placebo (conventional treatment only) in JIA in patients who either did not respond or did not tolerate MTX.
The basis for choosing LFN for patients in our study derived from reference books and clinical trials. Several articles have proposed this drug in the treatment algorithm of JIA [13, 14]. Also in the pediatric rheumatology reference, Petty 2021, LFN is mentioned for the treatment of JIA.
Material and methods
This study was a double-blind, placebo-controlled, randomized trial. No changes in methods were made after the study began. The sampling method used was purposive non-probability sampling.
Study population
Patients between the age of 2 and 20 years who were diagnosed with polyarticular or oligoarticular or extended oligoarticular subtypes of JIA, who were diagnosed before the age of 16 years, and who were unresponsive or had side effects to conventional therapy for JIA, including MTX, were included in this study.
The study population included patients who came primarily to the rheumatology clinic at Ghaem Hospital, or to the first author’s private service, or were referred by the co-authors.
Inclusion criteria
The inclusion criteria for the study are:
Children aged 2 to 20 years with a diagnosis of JIA based on the International League of Association for Rheumatology Classification of Juvenile Idiopathic Arthritis (ILAR) diagnostic criteria for JRA.
Suffering from polyarticular, extended oligoarticular and oligoarticular subtypes of JIA.
Resistance to conventional therapy of JIA:
presence of active arthritis despite treatment with MTX or combination therapies containing hydroxychloroquine, sulfasalazine, corticosteroids in addition to MTX (active arthritis was defined as the presence of more than two articular sites with swelling or tenderness or limitation of range of motion for at least the last 3 months since onset) or,
corticosteroid dependence that could not be tapered.
The presence of at least 3 joints with swelling or the presence of at least 3 painful joints with limited range of motion out of a total of 74 joints examined.
Exclusion criteria
Study exclusion criteria were:
Diagnosis of a JIA subtypes other than those listed in the inclusion criteria.
Diagnosis of other rheumatologic conditions that are collectively associated with JIA.
Patients who are unavailable or unable to consent.
Treatment with other biologic drugs, including etanercept, infliximab, or cytotoxic drugs 3 months prior to study enrollment.
Receipt of corticosteroids at a dose greater than 0.25 mg/kg/day 2 weeks prior to enrollment.
Receiving intravenous immunoglobulin (i.v. Ig) 2 weeks prior to study enrollment.
Presence of contraindications to the drugs used in this study, including active severe infections (e.g., sepsis or pneumonia).
Patients with more than a three-fold increase in serum alanine transaminase (ALT) or bilirubin compared to the upper normal limit, or a white blood cell count of less than 3,500/ml or a platelet count of less than 100,000/ml.
Patients with a history of macrophage activating syndrome.
Patients with a current diagnosis or history of malignancy.
Blinding and allocation
Interventions
Patients in the intervention group received LFN tablets orally for 3 months. Patients weighing less than 20 kg received 10 mg of LFN every other day. Patients weighing 20–40 kg received 10 mg of LFN daily, and patients weighing more than 40 kg received 20 mg of LFN daily.
Patients in the control group received oral placebo tablets, equivalent to LFN tablets at the same dosage as the intervention group. The placebo tablets were manufactured at the Faculty of Pharmacy, Mashhad University of Medical Sciences. The ingredients of the placebo tablets were starch and lactulose, which had no effect on the results of the study. The placebo tablets were manufactured to be visually similar to the LFN tablets.
Both the placebo and LFN tablets were placed in completely similar hard paper boxes that were uniquely coded and were prescribed to patients in each group. The investigator and patients were blinded to the contents of each box. Leflunomide and placebo tablets were administered to patients monthly.
In both groups, the conventional treatment with MTX and other DMARDs (hydroxychloroquine and sulfasalazine) was not stopped and their dose was equalized in both groups. In other words, LFN and placebo were added to the main treatment. Conventional therapy with MTX i.m. or p.o., was administered at a maximum dose of 6 mg/m2 weekly).
Since we were worried about the simultaneous use of two toxic and complicated drugs in children, we considered the dose of MTX to be lower in order to replicate the two groups. Patients in our region also did not tolerate high doses.
Patients were not allowed to receive biologic treatments or intra-articular corticosteroids for the duration of the study.
Outcomes
We evaluated response to treatment using the ACRPed response scale. American College of Rheumatology Pediatric response criteria developed in 1997 became the gold standard for the assessment of response to therapy in JIA. The most widely accepted criteria to define an improvement in patient disease course in response to a therapeutic intervention are ACR criteria developed in 1997.
These criteria are based on the ACR score outcome variables for juvenile arthritis, namely physician global assessment of disease activity Visual Analogue Scale (VAS), parent/patient assessment of overall wellbeing (VAS), functional ability, number of joints with active arthritis, number of joints with limited range of motion (ROM), and erythrocyte sedimentation rate (ESR). An ACRPed 30 response is defined as at least a 30% improvement from baseline in three of 6 variables, with no more than one remaining variable worsening by > 30%.
At the beginning of the study and at the end of each month (for a total of 3 months), patients were comprehensively examined by a rheumatologist, and the number of joints with active arthritis, pain, or limited range of motion was recorded separately for each patient.
The clinical assessment of the patient by the physician or the patient/parents’ assessment was recorded using a VAS. On this scale, the worst clinical situation is rated as 10 and the best as 0. The patient or examiner indicated the score on a 10 centimeter line, with the best situation representing the beginning of the line and the worst the end of the line. The scores were obtained with a ruler.
The most commonly reported adverse reactions to LFN are diarrhea, elevated liver enzymes, alopecia, and rash. All patients enrolled in this study were carefully evaluated for active infections, tuberculosis, and liver disease.
Therefore, laboratory tests were performed on each patient at baseline and at each monthly visit, including complete blood count (CBC), liver function tests (LFTs), ESR, C-reactive protein (CRP), serum levels of antinuclear antibodies (ANA), lipid profile, and tests for viral hepatitis as well as urinalysis.
In addition to laboratory tests, a complete medical history and physical examination were performed to identify signs and symptoms of possible treatment-related adverse events. All patients who exhibited signs or symptoms of possible serious adverse events were excluded from the study.
Statistical analysis
Statistical analyses were performed with the SPSS statistical package, version 11.5 (SPSS, Inc., Chicago, Illinois). Descriptive statistics were used to describe the characteristics of patients in each group. Categorical variables were presented as percentages and compared between two study groups using the χ2 test.
Continuous variables were reported as mean ±standard deviation or median (interquartile range). Considering the distribution status of continuous variables, Student’s t-test or the Mann-Whitney U test was used to compare groups at different time points of the study. The paired t test was also used to examine the time-dependent variables within the patient groups.
A 2-sided p-value < 0.05 was considered statistically significant. American College of Rheumatology Pediatric criteria 30% and ACRPed 50% for each patient were calculated using a formula developed specifically for these elements based on the 6 clinical criteria assessed in the study between the start of the study and week 12.
Bioethical standards
This trial was approved by the Ethics Committee of the Mashhad University of Medical Sciences with a registration number of 900527. This study is registered in the U.S. National Institutes of Health Registry of Clinical Trials (http://www.clinicaltrials.gov) with the registration number of NCT02024334. The parents of all subjects were informed about the details of the study and signed the study informed consent form.
Results
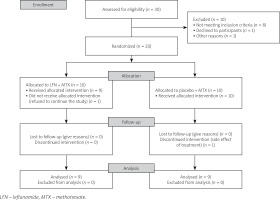
A total of 20 JIA patients participated in this study based on the inclusion and exclusion criteria. Patients were randomly divided into equal groups (n = 10) of intervention (MTX and LFN) and control groups. At baseline of the study one patient from the intervention group refused to continue the study and was excluded from the study.
Intervention was performed on the remaining 19 patients. During the study, one patient was excluded due to a more than threefold increase in liver enzymes as a side effect of treatment. Finally, 18 patients completed the study.
Baseline characteristics
Demographic characteristics of the intervention and control groups were not statistically different (Table I). Moreover, the intervention and control groups had a statistically similar disease duration (38.44 and 58.70 months respectively, p-value = 0.303).
Table I
Demographic characteristics of the study population
Clinical criteria of patients including number of active joints and joints with restricted range of motion, physician and patient global assessment, as well as CHAQ38 score and serum ESR level in both groups were not significantly different at the beginning of the study (Table II).
Table II
Clinical criteria of patients at the beginning of the study
Although the results of CHAQ38 were significant in the primary analysis between the two groups without considering the numerical difference in the duration of the disease, after adjusting the effect of the duration of the disease, this difference disappeared (p = 0.08).
Intervention outcomes
The clinical criteria of both groups were not significantly different at the end of the 4th and 8th weeks of treatment. However, CHAQ38 score was significantly higher in the intervention group at the end of the 12th week of treatment. Other clinical criteria were not significantly different at the end of the 12th week of treatment (Table III).
Table III
Clinical criteria at the end of weeks 4, 8 and 12
A general linear model was used to assess the response to treatment in all patients and whether the intervention and control groups were different. This statistical analysis evaluated the changes of the above-mentioned clinical criteria at the beginning, end of week 4, end of week 8 and end of week 12.
Physician and patient global assessments were the only variables that showed significant changes during the study period (p < 0.0001 and p = 0.001); however, the changes in physician global assessment were not significantly different between intervention and control groups (p = 0.137). Although a higher percentage of patients in the intervention group achieved ACRPed 30% and ACRPed 50%, the differences between groups were not statistically significant (Table IV).
Table IV
American College of Rheumatology Pediatric 30% and 50% response in leflunomide and placebo groups
| Item | Intervention | Control | p-value | ||
|---|---|---|---|---|---|
| Respondents | Non-respondents | Respondents | Non-respondents | ||
| ACRPed 30% | 4 (50%) | 4 (50%) | 3 (30%) | 7 (70%) | 0.630 |
| ACRPed 50% | 2 (25%) | 6 (75%) | 1 (10%) | 9 (90%) | 0.559 |
During the study, 4 patients reported gastrointestinal side effects such as abdominal pain and nausea at the end of week 4 and 3 patients reported these symptoms at the end of weeks 8 and 12 (Table V).
Table V
Reported side effects at the end of weeks 4, 8 and 12
| Side effects | End of week 4 | End of week 8 | End of week 12 | p-value | |||
|---|---|---|---|---|---|---|---|
| Leflunomide | Placebo | Leflunomide | Placebo | Leflunomide | Placebo | ||
| Gastrointestinal | 3 | 1 | 2 | 1 | 2 | 1 | 0.050 |
| p-value* | 0.257 | 0.999 | 0.999 | ||||
| Liver | 0 | 0 | 1 | 0 | 1 | 0 | 0.400 |
| p-value* | 0.999 | 0.999 | 0.999 | ||||
| Cutaneous | 0 | 0 | 0 | 0 | 0 | 0 | – |
| p-value* | – | – | – | ||||
Although more patients reported gastrointestinal symptoms in the intervention group, the differences between groups were not statistically significant. The most noteworthy side effect was a threefold increase in the level of liver enzymes in one patient in the intervention group at the end of week 8. This patient was excluded from continuing the study. The CONSORT diagram presents the sample entry process and attrition (Fig. 1).
Discussion
Juvenile idiopathic arthritis is the most common chronic inflammatory joint disease among children. It is associated with various complications including growth restriction and visual defects. Therefore, prompt treatment of JIA is important [16, 18]. Although MTX is known as the standard treatment for JIA, there are limited findings regarding the side effects of treatment and the response of the disease to this drug [18].
Based on some studies, its administration was associated with gastric intolerance and hematologic and hepatic side effects [15]. Leflunomide, also a DMARD, is recommended in patients with intolerance to MTX. There are few studies on the efficacy of LFN in JIA. To our knowledge, the effects of the combination of LFN and MTX in the treatment of JIA have not been fully assessed [15]. In this study, we compared the effect of LFN and MTX with that of MTX and placebo in the treatment of children with JIA using CHAQ38 and ACRPed 30% and 50%.
The results of the current study indicated that the majority of the variables improved in both groups. However, the improvement was more satisfactory in the MTX-plus-LFN group than in the control group. During the assessment periods, the pattern of change in the markers remained constant in the MTX-plus-LFN group while the extent of improvement started to decrease or reverse in the control group at week 12.
However, these changes were not statistically significant. An exception was the CHAQ38 score at week 12, which was higher in the intervention group compared to the control group. This finding was in line with previous studies in terms of the efficacy of both treatment methods. Nevertheless, the comparison between the studies revealed controversial results [12, 14, 19].
In the study by Ayaz [20], 36 patients with JIA, who were unable to receive MTX due to complications, were given LFN. In this study, unlike our study, there was no simultaneous administration of the two mentioned drugs. The results of this study demonstrated that LFN remained a valid alternative therapy for MTX intolerance or toxicity. Unlike our study, this study did not use a control group.
The best criteria to determine an improvement in response to a therapeutic strategy are ACRPed criteria. This criteria are based on physician global assessment of disease activity, parent/patient assessment of overall well-being, joint involvement and ESR [21].
The findings of our analysis showed that more patients in the intervention group achieved ACRPed 30% and 50% than in the control group. Gao et al. [12] reported that the average clinical improvement rate in the MTX-plus-LFN group was better than in the MTX group. Similarly, a multicenter study by Silverman et al. [10] showed that more patients in the MTX group reached ACRPed 30% at the end of 16 weeks compared to the LFN group. However, they reported no significant difference in the improvement index between groups [10].
The difference in the findings of previous studies and the current study might be due to the shorter follow-up time and smaller sample size in the current study. In order to increase treatment efficacy and to assess the primary outcome in JIA, we applied a combination of MTX and LFN. Although the effect of combination therapy was more prominent than that of single MTX treatment, this effect was not statistically significant in terms of achieving ACRPed 30%.
Our data show that both treatment methods resulted in improvement in patient and physician satisfaction. However, patients were significantly more satisfied in the combination group than in the control group.
The percentage of patients who achieved ACRPed 30% was higher in the MTX-plus-LFN group compared to the MTX group. The high level of response to treatment in both groups may be due to the fact that patients were in the early stage of the disease. Therefore, we suggest that combination therapy be considered before biologic therapy due to its effectiveness and safety.
Silverman et al. [10] reported that 52% of the patients achieved ACRPed 30% after 50 weeks of MTX and LFN treatment. This was slightly higher than the rate in the present study. This difference might be due to the small sample size and the shorter follow-up time in the latter study. Our findings are in line with a previous study that reported a significant clinical improvement with MTX and LFN compared to MTX alone [12].
This coherence was achieved between the studies regardless of differences in the assessment tools and inclusion criteria. While the current study included subjective and objective measures in patients with more diverse subgroups of JIA, the previous study only assessed clinical improvement in polyarticular JIA patients [12].
Based on our trial, combination therapy with MTX and LFN may only cause significantly different perceptions of improvement in patients or their caregivers compared to conventional MTX therapy. Overall, these findings were not fully confirmed nor rejected by the previous investigations due to the heterogeneous design, assessment and inclusion criteria of the studies.
Another possible reason for the difference in the findings of the studies might be the small sample size of the studies. Therefore, there is a need for larger multi-center trials to assess the effectiveness and safety of MTX and LFN co-administration in JIA patients.
Adverse effects
The current data revealed that the most common adverse effect in MTX and LFN co-administration was gastrointestinal symptoms followed by elevated liver enzymes. One case of severe side effect, a three-fold increase in liver enzymes, which occurred in the combination group, resulted in treatment cessation.
Nevertheless, these findings indicated non-significantly higher rates of side effects in the MTX and LFN group compared to the MTX group. Overall these findings demonstrated that the risk for severe side effects might be higher in combination therapy with MTX and LFN. In contrast to the findings of the current study, an additional trial on nearly twice the sample size revealed no side effects [12].
This difference might also be attributed to genetic and racial factors. These findings indicate the need for further studies with larger sample sizes to assess the safety of MTX plus LFN therapy in JIA.
Previous reports indicated that the commonly observed side effects of the combination therapy with LFN and MTX or LFN single therapy included gastritis and elevated liver enzymes [13, 22]. Elevated liver enzymes were also reported in patients who received adalimumab and colchicine besides LFN [10, 12].
The elevated liver enzymes resolved 2 weeks after cessation of LFN [12]. Since our team assessed the side effects of combination therapy in comparison to MTX single therapy, the results of the studies could not be definitely compared.
Study limitations
One of the strengths of this study was the inclusion of patients with different subgroups of JIA. This design provides a global picture of the efficacy and safety of MTX and LFN co-administration in JIA patients.
On the other hand, this diversity resulted in a small number of cases in each subgroup, which could not be compared using proper statistical methods. This could be considered as a limitation of this study, but this study did not intend to compare the effectiveness of the MTX and LFN treatment between different subgroups of JIA.
Therefore, it is recommended for further researchers to conduct studies on a larger sample of patients to assess whether JIA subtypes could be predictors for the effectiveness of combination therapy and incidence of side effects.
Conclusions
Based on the results of this study, adding LFN to conventional therapy yielded no significant difference in the outcome of JIA patients compared to MTX monotherapy. The results of this study also showed that combination therapy with LFN and MTX might increase side effects.
Therefore, patients treated with the combination therapy should be closely monitored for liver enzymes. These findings should be evaluated in larger studies before generating any recommendation or guideline.