Introduction
Knee osteoarthritis (OA) is one of the most common chronic diseases, affecting 24% of the adult population [1]. Diagnosis of knee OA relies on clinical [2] and radiological findings [3].
Treatment of OA is firstly based on a non-pharmacological approach such as weight loss, as recommended by EULAR [4]. Overweight seems to be correlated with OA even for immunologic factors other than the more evident biomechanical implications. Indeed, obesity and adipocytokines produced by systemic visceral adipose tissue and locally by the infrapatellar bursa, also known as Hoffa’s fat pad, might play an important role in the development and progression of knee OA [5].
Moreover, some adipocytokines (serum interleukin 6 and leptin) and synovial fluid leptin seem to be positively associated with body mass index (BMI) and preoperative pain severity in knee OA [6]. Second line treatment is based on topical and oral analgesic such as paracetamol, nonsteroidal anti-inflammatory drugs and symptomatic slow-acting drugs for osteoarthritis, followed by intra-articular (IA) viscosupplementation with hyaluronic acid (HA) [7].
For knee injections, both with corticosteroids and HA, two techniques are usually performed by physicians: the superolateral and the anterolateral approach. The superolateral approach, with the leg in extension, seems to be the most widespread and accurate injection technique [8]. The anterolateral approach is performed with a 90° flection of the knee, allowing the patient to remain in a sitting position, despite its lower accuracy [9].
In clinical practice, knee injection is a very well tolerated therapeutic option. In the literature, few papers have assessed injection related pain in knee OA, with contrasting outcomes between pain difference for the two techniques [10, 11].
The aim of this study was to evaluate differences of injection related pain using Visual Analogue Scale (VAS) [12], and the accuracy of the techniques in two groups of overweight patients, performing the anterolateral approach for one group and the superolateral approach for the second group.
Material and methods
We enrolled all overweight patients presenting to the Rheumatology Unit injection service of our hospital between September 2019 and March 2020. A total of one hundred twenty-six knees of 84 patients were divided in two groups, G1 (55 knees) and G2 (71 knees).
Inclusion criteria were knee osteoarthritis confirmed by clinical and radiological evidence (Kellgren-Lawrence score ≥ 2) [3] and BMI ≥ 25. Exclusion criteria were bleeding disorders, knee effusion, joint prothesis. Moderate molecular weight HA (range 1500–3000 kDa) was injected [13]. Pain during injection was evaluated using the VAS for pain (0–10), where 0 indicates no pain and 10 indicates the most severe pain. Before injection, meticulous aseptic technique was performed.
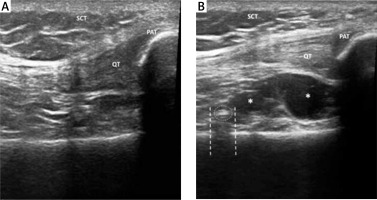
Procedure accuracy was evaluated by ultrasound (US). Ultrasonography was performed with an Esaote MyLab. Twice machine equipped with a multifrequency 6–18 MHz linear probe. Ultrasound examination permitted the direct visualization of viscous hyperechoic fluid, often associated with hyperechoic foci and air bubbles, spreading into the joint capsule of the suprapatellar recess [14] (Fig. 1).
Fig. 1
Ultrasound examination performed before IA injection demonstrating absence of fluid in suprapatellar recess (A). Ultrasound examination performed after IA injection demonstrates a small amount of hypoechoic fluid (*) in the suprapatellar recess (B). Some hyperechoic foci (dotted circle) with mild acoustic posterior shadow (dashed line) are often visible after IA HA injection, because of the viscous nature of HA and the presence of bubbles. SCT – subcutaneous tissue, QT – quadricipital tendon, PAT – patella.
SCT – subcutaneous tissue, QT – quadricipital tendon, PAT – patella.
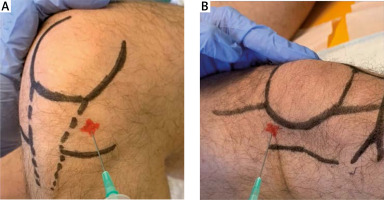
The suprapatellar knee recess was scanned with an anterior longitudinal approach, proximal to the superior pole of the patella. The anterolateral approach was used for G1. The superolateral approach was used for G2. For all the injections, 21-gauge needles were used. The anterolateral access (G1) was performed with the patient in a sitting position with the knee flexed to 90° at 1 cm proximal to the joint line, lateral to the patellar tendon, directing the needle towards the inter-condylar notch (Fig. 2 A). The superolateral access (G2) was performed with the patient in supine position, extending the knee; the needle was inserted 1 cm above and 1 cm lateral to the superolateral margin of the patella at a 90° angle to the femoral condyle with a concomitant subluxation of patella (Fig. 2 B). Both techniques were executed with no US aid and performed by the same trained physician.
Statistical analysis
The Mann-Whitney test for unpaired data was used to compare the VAS pain score between G1 and G2. Spearman’s test was performed to assess the correlation between VAS pain score and BMI, age, gender, and site of injection. Linear regression was performed for each access to evaluate the relationship between VAS pain score and the independent variables.
Results
A total of 126 knees of 84 patients were evaluated. Twenty-six patients were male, 58 female. Mean age was 69.9 ±9.01. Mean VAS pain score for the anterolateral approach (G1) was 1.71 ±1.89. Mean VAS pain score for the superolateral approach (G2) was 1.74 ±1.31. Mean BMI was 29.69 ±2.86. Mean BMI of G1 was 28.29 ±3.29. Mean BMI of G2 was 30.32 ±2.41.
No adverse events (AE) occurred in either group. The accuracy rate of the procedure, evaluated by US after the injection, was 69.1% for G1 (38/55 knees), 95.7% for G2 (68/71 knees). No significant difference was found in VAS pain score between G1 and G2 (p = 0.45). We found a significant correlation between BMI and VAS pain score in anterolateral accesses (G1) (r = 0.51; p < 0.005). No correlation was evidenced between age and VAS pain score in anterolateral access (G1). For the superolateral access (G2), no correlation was found for age or BMI and VAS pain.
Demographic and clinical characteristics of our cohort are reported in Table I.
Table I
Demographic and clinical characteristics of patients
Discussion
We found no difference in injection related pain between anterolateral and superolateral access of knee injection of HA in overweight patients. An increase in BMI seems to be a predictor of pain in anterolateral access. Our pain VAS results are partially in contrast with previous findings: Chavez-Chiang et al. [10] reported a mean VAS pain score of 4.8 for anterolateral access, while Lee et al. [11] found that the anterolateral approach is less painful than superolateral. We could hypothesize that such differences might be related to the use of a thinner 21-gauge needle than Lee’s cohort [11]; moreover, operator expertise and grade of local joint inflammation may be taken into account in order to explain the different outcomes.
We assessed the relation between BMI and pain in anterolateral access. This evidence could be explained locally, with a major production of adipocytokines [5] partially due to the plentiful of subcutaneous tissue in overweight patients rather than Hoffa’s fat pad, whose volume seems not related to weight [15]. The influence of meniscal lesions should be considered too, since adipocytokines may have an influence on its pathogenesis [16].
We found an accuracy rate, evidenced by US, of 95.7% for superolateral access, while a lower rate of 69.1% was evidenced for anterolateral access, as recently reported [8]. This finding could be explained by the different and complex anatomical structure between the anterior and lateral compartment of the knee, which might even present differences between both knees of the same patients [17].
Finally, in relationship with the different knee compartments it is possible to speculate that HA may not have a homogeneous distribution in the whole joint [18]. In our cohort, no patients reported AEs, confirming the safety of HA injection [19].
Conclusions
Hyaluronic acid injection is a widespread and safe therapeutic option for knee OA with no significant differences between anterolateral and superolateral approaches in terms of pain in overweight patients. However, higher BMI seems to be a predictor of pain in anterolateral access, and the superolateral approach should be preferred for its accuracy.