Introduction
Systemic sclerosis (SSc) is a generalized chronic connective tissue disorder of unknown etiology [1]. Diffuse fibroproliferative vasculopathy is one of the critical abnormalities in scleroderma most commonly involving the microvasculature system, leading to Raynaud’s phenomenon and digital ischemia [2]. Nailfold capillaroscopy is a non-invasive method that is increasingly used in the evaluation of patients with Raynaud’s phenomenon and scleroderma spectrum of diseases [3, 4].
Capillary changes in patients with developing or established SSc are drop-out of the capillaries, enlargement of the capillary loops, microhemorrhages, neo-angiogenesis and changes in the capillary morphology and architecture [5]. There are multiple studies on the relationship of the severity of capillary findings and clinical manifestations and some poor prognostic manifestations of patients with SSc, such as pulmonary hypertension and digital ulcers [6–9].
Since systemic sclerosis is a rare disease, most of the data regarding ocular involvement come from single case reports or studies with a small number of samples [10–13]. Some of the reported features in SSc are well known, such as eyelid skin changes, dry eye and astigmatism, but others are not well understood, e.g. scleroderma retinopathy, although choroidal circulation disorders have been reported in more than 33% of SSc patients in some studies, e.g. that of Waszczykowska et al. [14]. A recent review of the literature by Ushiyama et al. showed a prevalence of 34% for retinal changes [12].
In SSc, small vessels are mostly affected and, in some studies, cotton-wool spots in the retina, frequent atrophic lesions of the retinal pigment epithelium within the posterior pole of the retina, dilatation of the venous vessels, intra-retinal extravasation, hard exudates, and macular edema have been reported [10, 11, 14–16].
In a few studies, some evidence of the relationships between nailfold capillaroscopy and retinal changes has been reported. Gomes et al. [10] found that retinal microvascular abnormalities are indistinguishable from those related to systemic hypertension in patients with SSc and they are associated with severe capillaroscopic patterns; however, they did not find any relationship between eyelid skin changes, dry eye and capillaroscopy patterns in patients with SSc. In a study by Ushiyama et al., there was no relationship between retinal vascular changes and nailfold capillary damage [12].
In this study, we evaluated the retinal changes including vascular tortuosity, hemorrhage and hard exudates in patients with SSc using fundus photography and retinal examination by an ophthalmologist. In addition, we searched for any association between nailfold capillaroscopy and retinal vascular changes and aimed to determine whether we could use capillaroscopy as a predictor of retinal involvement in patients with SSc.
Material and methods
The authors included 52 adult patients with SSc who fulfilled the 2013 ACR/EULAR SSc diagnostic criteria [17]. They actively participated in the study and referred to the clinic of scleroderma of Hafez Hospital or were admitted to the Rheumatology Ward of Hafez Hospital affiliated to Shiraz University of Medical Sciences, in the period from January 2016 to January 2017, with an age range of 16–65 years.
The exclusion criteria were cases with overlap syndrome, diabetes mellitus, current smokers and patients with hypertension. In addition, patients with a history of previous ophthalmic surgery, any retinal pathology proven due to known diseases not related to scleroderma, intraocular inflammation, ocular hypertension, previous history of uncontrolled hypertension or renal crisis and a history of ocular trauma were excluded.
The sub-investigators received a checklist with inclusion and exclusion criteria. When a subject met the inclusion criteria while having no exclusion criteria, the data were registered.
The authors obtained the consent of the bioethical commission to conduct the present study.
All the patients signed a declaration of consent, before being included in the study population. All clinical manifestations of patients and their organ involvement were recorded during evaluation.
The rheumatologist involved in this research project performed the physical examination.
The demographic data including disease duration (date of first Raynaud’s and non-Raynaud’s symptoms); serological (anti-Scl-70, anti-centromere and antinuclear antibody), clinical [diffuse cutaneous SSc (dsSSc), and limited cutaneous SSc (lcSSc)] subsets according to LeRoy et al. [1]; and skin scoring according to the definition by LeRoy et al. in the MRSS (Modified Rodnan Score) [18] classified into 5 groups – normal, mild (1–14), moderate (15–29), severe (30–39) and end stage (40+) – were gathered. Pulmonary involvement was assessed by high resolution CT scan (HRCT) at the first screening or after detecting low diffusing capacity of lung for carbon monoxide (DLCO) < 80% or low forced expiratory volume (FEV1) < 75% in the pulmonary function test (PFT).
On the same day, the patients with SSc underwent capillaroscopy by stereomicroscope Euromax ST. 1740 at 250 W power and the video camera Cmex D.C. 5000 (5 megapixels). Immersion oil was applied on the nail-fold bed to improve resolution. Eight fingers of the two hands excluding the thumbs were examined. We also took at least one image of the middle of nailfolds per finger, and the data were analyzed by a rheumatologist involved in this study.
The capillaroscopic data including capillary distribution (disturbed or normal), shape of capillaries (normal hairpin, subtle change including tortuosity and crossing, anomalies including meandering, ectasia, neo-angiogenesis and bizarre capillaries), the largest diameter of the arterial or venous side [dilated loops were defined as increase of capillary diameter ≥ 20 and < 50 μm) and giant capillaries (a capillary with a homogeneously enlarged loop with a diameter ≥ 50 μm)], capillary length (normal or elongated: ≥ 300 μm), mean capillary density (capillary loss defined as reduction of the normal number of capillaries below 7 per linear millimeter), avascular area (intercapillary distance > 500 μm): present or absent, microhemorrhages: present or absent, and neoangiogenesis or ramified capillaries: present or absent, were recorded in a form [19].
The whole findings were defined as normal, specific scleroderma changes for early, active, and late scleroderma pattern, or non-specific abnormalities based on recommendation of Cutulo et al. [20].
ESR was measured by the Westergren method. Serum CRP by nephelometry; RF by latex test; ANA and anticentromere Ab or anti-Scl70 by the ELISA method were checked.
The patients who fulfilled the inclusion criteria were referred to the ophthalmology clinic of Poostchi to undergo noninvasive fundus color photography. The fundus photography was done using the fundus camera TRC 50 EX (Topcon) following pupil dilatation with 1% tropicamide solution (eye drops) and then the patients were referred to Motahari clinic where retinal evaluation was done by a board certified and expert posterior segment ophthalmologist blinded to rheumatologic manifestations of patients. For each participant, a full ophthalmic examination, including best corrected visual acuity, slit-lamp examination, tonometry, and fundoscopy, was done. The important findings in the retinal examination were the existence of any exudate, hemorrhage and vascular morphological changes. Findings were reported qualitatively.
Statistical analysis was done using SPSS-19 and descriptive statistics and χ2 and t-test. The level of p < 0.05 was considered significant.
Results
The authors included 52 patients in the study. The age range of the study population was 20 to 62 years (mean age: 40 ±9 years). Among the study participants, 3 (5.8%) were male and 49 (94.2%) were female. Their mean disease duration was 8 ±5 years. There were 35 patients with limited and 17 with diffuse scleroderma. The clinical, laboratory tests and other details of capillaroscopy findings are also presented in Table I.
Table I
Demographic, capillaroscopy, skin score, clinical manifestations and laboratory features of patients and their association with types of SSc
In ophthalmologic examination by an ophthalmologist: visual acuity was below 0.8 in 9 (17%) patients, lid thickness was reported in 3 (5.7%) patients, dry eye was reported in 8 (15.3%) patients, cup to disc ratio was below 50% in all patients, mean ocular pressure was 14.05 ±2.74, and three (5%) patients had cataract. The limbus, cornea, iris and anterior chamber were normal in all patients. Retinal vascular changes in ophthalmologic examination were seen in 30% of patients as increased vascular tortuosity (mainly in large vessels that could be seen in examination). Fundus photography that was checked by the same ophthalmologist in 40 patients revealed that 22.5% had vascular tortuosity (52 patients were examined by the ophthalmologist and 40 of them underwent photography of the fundus). None of the patients had cotton wool spot, hemorrhage or hard exudate in retinal examination.
Then, we compared the association of abnormal vascular finding defined as increased vascular tortuosity in the ophthalmologist examination and fundus photography with capillaroscopy components of the patients’ nailfolds, disease duration, and skin score of patients, as shown in Table II. Figure 1 presents eye fundus photographs and simultaneous nailfold capillaroscopy changes.
Table II
Association of capillaroscopy findings with retinal vascular changes in ophthalmologist examination and digital fundus photography
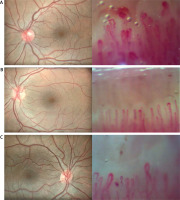
Fig. 1
Eye fundus photographs and simultaneous nailfold capillaroscopy changes. Normal fundus photography with increased tortuosity of the vessels and at the same time nailfold capillaroscopy that shows multiple homogeneously dilated capillaries, multiple microhemorrhages and severe disturbed distribution of capillaries (A), normal fundus photography and simultaneous multiple capillaries with apical dilatation and rare microhemorrhages (B), normal fundus photography and simultaneous multiple homogeneously dilated capillaries and a few microhemorrhages in capillaroscopy of nailfolds (C).
The capillaroscopic findings had no association with retinal findings, except for capillary hemorrhage in capillaroscopy, which was related to vascular tortuosity reported in ophthalmoscopy (p = 0.014).
Type of scleroderma, skin score and disease duration had no association with retinal findings (p = 0.882, 0.670 and 0.622), respectively.
Discussion
In this study, the authors evaluated the prevalence of retinal vascular changes such as vascular tortuosity in patients with scleroderma. We also compared the retinal changes with capillaroscopy findings, type of scleroderma, duration of the disease, and skin score.
In the study of complete eye examination, 17% of our patients had decreased visual acuity. In another study by Waszczykowska et al. [14] on patients with SSc, there was no significant difference between the patients and control population in visual acuity. In addition, we found a low frequency of increased lid thickness that was reported in 3 (5.7%) patients and also dry eye that was seen in 8 (15.3%) patients. Cup to disc ratio was normal in all of our patients and mean ocular pressure was 14.05 ±2.74. Three (5%) patients had cataract. The limbus, cornea, iris and anterior chamber were normal in all patients.
Compared to other studies which used the Schirmer test for detection of dry eye, our patients mostly had normal intraocular pressure and a lower percentage of dry eye, while other authors reported a rate of 49% for dry eye in their patients, 42.2% for cataract, and about 13% for glaucoma [10].
Our study focused mostly on retinal changes. We found retinal vascular changes presenting as increased microvascular tortuosity in about 30.7% and 22.5% of the study participants in our ophthalmology examination and fundus photography, respectively.
Ushiyama et al. [12] reported the prevalence of 34% for retinal vascular abnormalities including hard exudate, vascular tortuosity and macular degeneration in their patients with SSc. Choroid vasculature seemed to be more involved than retinal circulation in the systematic review by Kreps et al. [15]. In another study, by Jakhar et al. [21], prevalence of retinal changes was 28.89%.
The prevalence of retinal vascular changes is in line with the results of our study, indicating a high prevalence of retinal vascular changes among scleroderma patients. Ushiyama et al. [12] reported a prevalence of 8% in the control group. In a recent study performed by Szucs et al. [22] 58.9% of the patients had retinal and choroid changes found in the photography of the fundus.
In addition to specific pathologic alterations in retinal vasculature in the scleroderma, other factors may play a role in retinal vascular changes in scleroderma patients. These patients have a high prevalence of hypertension and hypertension crisis. Ushiyama et al. [12] reported higher systolic blood pressure and age in the group of scleroderma patients in comparison to the control group. Szucs et al. [22] did not exclude hypertensive patients. In our study, we excluded patients with diabetes mellitus and smokers, and we did not have any patient with a history of hypertensive crisis. Therefore, these etiologies have no role in the prevalence of retinal vascular changes in our study.
Aissopou et al. [23] found no difference in retinal vasculature of patients with SSc in comparison to the control group. In this study, the participants were non-diabetics with no history of hypertension and antihypertensive treatments. They concluded that the changes in vasculature in patients with SSc may be different in mechanism and severity compared with the retina and other parts of the body. Hypothetically, these characteristics may provide sparing of the retina from the “fibrous” vasculopathy seen in other organs of the body [23].
In our study, we found no significant relationship between scleroderma type and retinal vascular changes consisting of increased tortuosity. Duration of the disease and skin score had no association with retinal changes (all p > 0.05). Similar findings were reported by Ushiyama et al. [12].
These findings indicate that the mechanisms of changes in retinal vasculature and nailfold vasculature may be at least partly different. Therefore, the changes in retinal vasculature and nailfold vasculature cannot be used for prediction of each other’s prevalence. In addition, the findings support the conclusion of Aissopou et al. [23] that fibroproliferative vasculopathy in patients with SSc may depend on specific cellular or soluble factors not present in the retinal environment.
Based on the results of our study, duration of the disease had no effects on venular tortuosity in gross evaluation by the ophthalmologist and fundus photography. Aissopou et al. [23] also found that retinal vascular involvement in scleroderma was unrelated to disease duration.
In this study, skin score was not significantly different in scleroderma patients with different involvement of retinal vasculature. Similar to our findings, no relationship between retinal vascular changes and the extent of skin involvement was reported by Aissopou et al. [23].
We found increased retinal vascular tortuosity in one third of our patients in examination of the eye by the ophthalmologist and 22.2% by fundus photography. We did not find any parallel correlation between retinal tortuosity and the nailfold capillary patterns, except for hemorrhage, and possibly it was based on different mechanisms and functions of microvasculature in these different areas of the body. It is known that the nailfolds have a high density of arteriovenous anastomoses, which have direct connections between the arterioles and venules with no nutritional function, but instead these anastomoses function as thermoregulators. These sites are distinct from other skin areas due to their specialized structures and functional features for thermoregulation [24].
A previous study by Shenavandeh et al. [25] on the correlation of ear involvement and capillaroscopy of SSc patients also did not show any correlation between nailfold-capillary changes and ear involvement, so it seems the pathogenic mechanisms in organs such as the ear and eye are different from nailfold capillaries in these patients.
We did not use angiography of the retina, as we did not try to use any invasive tool in our asymptomatic patients; so, this was one of our study limitations. Retinal angiography is probably more accurate than gross physical examination and fundus photography. Our retinal study was based on the examination of the ophthalmologist and fundus photography that was seen grossly, and the diameters and characteristics of the vessels were not calculated in detail and only grossly interpreted. In addition, the other limitation of our study was the lack of comparison with the control group, which should be done in future studies.
Conclusions
Retinal vascular changes defined as vascular tortuosity were observed in one third of scleroderma patients by ophthalmologist examination and slightly lower in the fundus photography. There was no significant difference in the retinal vascular changes and skin score, capillaroscopic characteristics, and disease duration, except for the patients who had hemorrhage in nailfold capillaries. These findings indicate that we need to conduct studies on pathologies in smaller vessels of the eye.
Although nailfold capillaroscopy at present cannot predict the retinal changes in scleroderma patients, advances in our retinal examination techniques to obtain information about smaller vascular structures will help us in the future to predict the development of retinal changes by nailfold capillaroscopy and instigate early treatment for retinal involvement.



