Introduction
Ankylosing spondylitis (AS) is a disease primarily affecting the spine and sacroiliac joints, which is characterised by inflammatory lower back pain, enthesitis and new bone formation. The prevalence of AS is approximately 0.1–1.4% and it is seen in males 2–3 fold more frequently than in females.
The age of onset is younger than 30 years in 80% of cases and < 5% of cases have onset after the age of 45 years. Enthesitis is inflammation of the attachment site of the tendons, ligaments and the joint capsule to the bone, and it is a characteristic feature of seronegative spondyloarthropathies (SpA). Enthesitis can be determined clinically in 10% of patients with early stage AS, and in 50% of advanced stage AS patients [1].
Periodontal disease is the general term for chronic inflammatory diseases of the tissues around the tooth. The periodontal tissues include the gingiva, alveolar bone, cement and the periodontal ligament, which has a supportive function attaching the tooth to the bone.
This function is provided by the basic fibres of the periodontal ligament, which form a strong connection between the cement and the bone. The most important part of the periodontal ligament is formed of the principal fibrils, which are attached at one end to the bone and at the other end to the dental cement with Sharpey fibres [2].
The prevalence of periodontitis shows variation from country to country, but it has been reported to affect 20–50% of the global population [3]. The aetiology of periodontitis is multifactorial, with known risk factors of smoking, age, diabetes mellitus, education level, gender and immunological diseases such as human immunodeficiency virus (HIV) [3–5]. Many studies have shown a strong relationship between rheumatoid arthritis and periodontitis [6].
The aim of this study was to evaluate the periodontal status of patients with AS and to determine the factors affecting this relationship.
Material and methods
The study included 200 patients diagnosed with AS according to the modified New York criteria [7] and followed up in the rheumatology policlinic. Patients were excluded from the study if they were pregnant, had diabetes, or were aged < 18 years. The study protocol was approved by the Akdeniz University Ethics Committee (Project No. 3918).
A record was made for all the patients of age, gender, body mass index, comorbidities, smoking status, disease duration and drug treatments. The patients were separated into two groups: those taking non-steroid anti-inflammatory drugs (NSAID) and those taking anti-tumour necrosis factor drugs (anti-TNF). Disease activity was evaluated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), mobility with the Bath Ankylosing Spondylitis Metrology Index (BASMI), functional status with the Bath Ankylosing Spondylitis Functional Index (BASFI), enthesitis with the integrated Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), and quality of life with the Ankylosing Spondylitis Quality of Life (ASQoL) scale. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values were recorded from the hospital information system.
Periodontal examinations were conducted in the periodontology clinic. The periodontal condition was thoroughly assessed using the clinical and radiographic criteria stated and defined according to the American Academy of Periodontology guidelines [8].
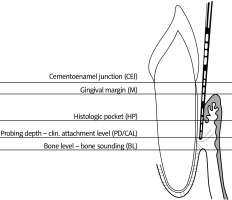
Full-mouth clinical periodontal measurements were recorded at six sites per tooth (mesio-buccal, vestibular, disto-buccal, disto-lingual, lingual, mesio-lingual), and the plaque index (PI) [9], gingival index (GI) [10], bleeding on probing (BOP), which was recorded as present or absent, probing depth (PD) and clinical attachment level (CAL) were recorded.
A Williams periodontal probe (Williams, Hu-Friedy, Chicago, IL, USA) was used for the clinical periodontal measurements, which were all performed by the same examiner. Probing depth (PD) was defined as the distance from the free gingival margin to the bottom of the sulcus or periodontal pocket.
Gingival recession was defined as the distance from the cemento-enamel junction (CEJ) to the free gingival margin. Clinical attachment level was defined as the distance from the cemento-enamel junction to the bottom of the sulcus or periodontal pocket and was calculated as the total of the PD and gingival recession measurements [11] (Fig. 1).
Statistical analysis
Data obtained in the study were analysed statistically using SPSS v. 25.0 software. Descriptive statistics of quantitative data were stated as mean and standard deviation (SD) values and categorical data as number (n) and percentage (%).
Conformity of the data to normal distribution was assessed with the Shapiro-Wilk test. As the data did not show normal distribution, non-parametric tests were used. Correlation analysis was applied to examine the relationships between variables. A value of p < 0.05 was accepted as statistically significant.
Results
Evaluation was made of a total of 200 patients, as 71 (35.5%) in the NSAID group and 129 (64.5%) in the anti-TNF drug group. The mean age of patients in the NSAID group and anti-TNF drug group was 39.39 ±10.1 and 40.12 ±10.1 years, respectively (p = 0.710).
The non-steroid anti-inflammatory drugs group comprised 30 (42.3%) females and 41 (57.7%) males, and the anti-TNF drug group comprised 27 (20.9%) females and 102 (79.1%) males. There were 26 (36.6%) patients who smoked in the NSAID group and 52 (40.3%) in the anti-TNF group, with no statistically significant difference determined. Human leukocyte antigen (HLA) B27 positivity was determined in 63.4% of the NSAID group and 76.9% of the anti-TNF group.
Periodontitis was determined in 26 (36.6%) patients of the NSAID group and in 45 (34.9%) of the anti-TNF group, and the difference was not determined to be statistically significant. The time since diagnosis and duration of symptoms were longer, and BASMI, BOP and GI values were determined to be statistically significantly higher in the anti-TNF group.
Morning stiffness, MASES, BASDAI, ASQoL and CRP values were determined to be statistically significantly higher in the NSAID group. No difference was determined between the groups for the other parameters (Table I).
Table I
Disease and treatment profile in patients with ankylosing spondylitis
[i] ASQoL – Ankylosing Spondylitis Quality of Life, BASDAI – Bath Ankylosing Spondylitis Disease Activity Index, BASFI – Bath Ankylosing Spondylitis Functional Index, BASMI – Bath Ankylosing Spondylitis Metrology Index, BMI – body mass index, BOP – bleeding on probing, CAL – clinical attachment loss, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, GI – gingival index, MASES – Maastricht Ankylosing Spondylitis Enthesitis Score, NSAID – non-steroidal anti-inflammatory drug, PD – pocket probing depth, PI – plaque index, TNF – tumour necrosis factor.
A statistically significant correlation was determined between periodontitis and age (p = 0.007), BASFI (p = 0.024), BASMI (p = 0.001), PI, PD, CAL, BOP, and GI (p = 0.000). The mean age was 42 years in patients with periodontitis and 38 years in those without periodontitis. In the patients with periodontitis, the BASFI score was mean 3.2, BASMI: 4.2, PI: 2, PD: 2.9, CAL: 3.1, BOP: 0.4, and GI: 1.6.
In the patients without periodontitis, the BASFI score was mean 2.3, BASMI: 3.6, PI: 1.5, PD: 1.8, CAL: 1.9, BOP: 0.2, and GI: 1.2.
A correlation was determined between MASES and BASDAI, BASFI, ASQoL, BOP, and GI values. The bleeding on probing and GI values were determined to be higher in the anti-TNF group (Table II).
Table II
Disease activity scores, clinical, laboratory and periodontal parameters in ankylosing spondylitis
[i] ASQoL – Ankylosing Spondylitis Quality of Life, BASDAI – Bath Ankylosing Spondylitis Disease Activity Index, BASFI – Bath Ankylosing Spondylitis Functional Index, BASMI – Bath Ankylosing Spondylitis Metrology Index, BMI – body mass index, BOP – bleeding on probing, CAL – clinical attachment loss, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, GI – gingival index, MASES – Maastricht Ankylosing Spondylitis Enthesitis Score, PD – pocket probing depth, PI – plaque index.
Discussion
Various studies have determined periodontal disease at a higher prevalence in patients with AS than in the healthy population [12–19].
Keller et al. [18] reported periodontal disease in 45% of AS patients and in 25.9% of a corresponding control group. Higher bacterial prevalence in AS patients has been associated with inadequate oral hygiene. In animal studies, a relationship has been found between HLA-B27 and alveolar bone loss [20].
Agrawal et al. [15] hypothesised that HLA-B27 could have a role in the interaction between AS and aggressive periodontitis. However, this hypothesis was based on a single case report of an AS patient with HLA-B27 positive aggressive periodontitis. That study also showed no relationship between HLA-B27 and periodontal findings such as CAL, PD and BOP [15].
In the current study, no significant difference was determined between HLA-B27 positive and negative patients with respect to periodontitis. In another study conducted on Caucasians, the sensitivity of HLA-A9 and B15 to aggressive periodontitis was determined to be increased [21].
Serum IL-2, IL-6 and TNF-α levels are high in AS patients [22]. It has been determined that in periodontitis, pro-inflammatory cytokines such as IL-1, IL-2, IL-6, IL-17 and TNF-α in the gingival pocket fluid are increased, and cytokine levels decrease after periodontal treatment [23–27].
In a study by Fabri et al. [16], although there was an evident improvement in the periodontal parameters of AS patients following anti-TNF treatment, this improvement was not observed in RA patients. It is not clear whether periodontitis is a result of AS disease or whether it is more effective in the pathogenesis, as in RA.
Similarly, Erciyes et al. [28] determined that the periodontal health of AS patients was worse than that of a control group. It was reported that as similar pro-inflammatory cytokines have a role in both diseases, the course of both could be affected.
Ratz et al. [12] conducted a meta-analysis of 6 studies which compared periodontal measurements of AS patients and control cases. A positive correlation was seen between AS and the severity of periodontitis in all the studies, but it was only statistically significant in two studies.
Although there was no significant difference with respect to CAL and PD, the BOP values were significantly higher in the AS patients (p = 0.0005) [11]. In the current study, the BOP and GI values were higher in the anti-TNF group than in the NSAID group.
In a previous study in which plaque samples were taken from axial SpA patients, although periodontitis prevalence was higher, no difference was seen compared to the control group with respect to microbial variety [29]. While the contribution of periodontitis to the pathogenesis of RA is clear, this has not yet been clarified for AS.
In a study by Kang et al. [13], a significant relationship was determined between periodontitis and reduced mobility in AS patients. In the current study, a significant relationship was determined between BASFI and BASMI values and periodontitis.
In a large cohort study of 6428 patients diagnosed with psoriatic arthritis (PsA) in Denmark, the incidence rates of periodontitis in the PsA patients were found to be significantly higher than in the reference population. When psoriatic arthritis patients were compared with patients with psoriasis only, the rate of periodontitis was determined to be higher [30].
Enthesis is a basic finding of AS and there is therefore a need for further studies to investigate whether periodontitis can be considered as an enthesis. More frequent periodontitis in PsA than psoriasis may support enthesitis.
In the current study, the MASES scores of both the NSAID and anti-TNF groups were low, but were determined to be lower in the anti-TNF group. The drugs used by both groups are known to be extremely effective in the treatment of enthesis.
Previous studies have shown that NSAIDs and anti-TNF drugs provide improvement in periodontitis [31–33]. Therefore, as the current study patients were all using NSAIDs or anti-TNF drugs, the periodontitis and measurements could have been masked.
In a study by Iordache et al. [34], there was shown to be a significant improvement in disease activity, inflammatory parameters and periodontal status with 24 hours after starting anti-TNF drugs without applying periodontal treatment in AS patients.
Ancuta et al. [35] also showed a significant improvement in disease activity and periodontal status in PsA patients at 6 months after starting anti-TNF drugs.
Białowąs et al. [36] reported that despite reduced disease activity with periodontal treatment in RA, there was no improvement in clinical and biochemical parameters in SpA patients. This shows that while the contribution to disease pathogenesis in RA is more evident, there may be a relationship with enthesis in AS.
In spondyloarthropathies group diseases, the periodontal area may be an enthesis region and therefore periodontitis may be an enthesis. Anti-TNF treatments are known to be effective for enthesis and thus the benefit of anti-TNF drugs, even without periodontal treatment, can be evaluated as part of the treatment of systemic inflammation.
Conclusions
In conclusion, when the results of the current study are examined, although a relationship was seen between AS and periodontitis, there were the limitations of the cross-sectional design of the study and the lack of a control group. In spondyloarthropathies, periodontitis may be a component of the disease, in other words an enthesis.
No opinion that periodontitis could be an enthesis could be found in the literature. There is a need for further studies on this subject. Patients must be evaluated with respect to periodontitis in SpA group diseases.