Introduction
Ankylosing spondylitis (AS), characterized by chronic inflammation and pathological new bone formation at axial joints, is thought to develop and persist due to aberrant immune reactions at sites of mechanical stress and interactions between innate and adaptive immune mechanisms [1].
The association of AS with HLA-B27 and endoplasmic reticulum aminopeptidases (ERAP) encoding alleles points to antigen presentation and T-cell-mediated adaptive immunity as critical contributors to disease pathology [2, 3].
This is supported by reports showing numerous T-cells infiltrating affected joints in early and active sacroiliitis [4, 5] along with an increased number and greater proliferation of CD4+ and CD8+ T-cells in synovial fluid and peripheral blood of AS patients [6–9].
Mesenchymal stromal/stem cells (MSCs) possess immunomodulatory potential and, among other abilities, can inhibit proliferation of T-cells [10, 11] and decrease the level of proinflammatory cytokines [12].
However, their functionality in AS patients is relatively unknown. Scarce data indicate aberrant functions and reduced immunomodulatory potential of bone marrow-derived MSCs (BM-MSCs) [13–15].
Moreover, we have previously found that adipose tissue-derived MSCs (ASCs) of AS patients (AS/ASCs) show certain abnormalities in the expression of surface markers and secretory activity [16]. Thus, it is possible that immunomodulatory capabilities of these cells may also be impaired.
To clarify this, we presently assessed direct anti-proliferative effects of AS/ASCs on allogeneic T lymphocytes, using ASCs from healthy donors (HD/ASCs) as a control.
To this aim, the proliferation of purified activated CD4+ T-cells co-cultured with ASCs was evaluated by several indices, and the contribution of soluble factors, i.e. kynurenines, prostaglandin E2 (PGE-2), interleukin 10 (IL-10), and interleukin 1 receptor antagonist (IL-1Ra), to ASCs-triggered effects was also analysed.
Material and methods
Patients, sample collection, and ethics approval
A group of 11 patients (6 female, 5 male) who fulfilled the Assessment of SpondyloArthritis International Society (ASAS) criteria for AS [17] were included in the study. Demographic and clinical characteristics of the patients are presented in Table I.
Table I
Demographic and clinical characteristics of patients
[i] Except where indicated otherwise, values are the median (range). ASDAS – Ankylosing Spondylitis Disease Activity Score, BASDAI – Bath Ankylosing Spondylitis Disease Activity Index, BASFI – Bath Ankylosing Spondylitis Functional Index, BASMI – Bath Ankylosing Spondylitis Metrology Index, CRP – C-reactive protein, DMARDs – disease-modifying anti-rheumatic drugs, ESR – erythrocyte sedimentation rate, HAQ – Health Assessment Questionnaire, NSAIDs – non-steroid anti-inflammatory drugs.
This study meets all criteria contained in the Declaration of Helsinki and was approved by the Ethics Committee of the National Institute of Geriatrics, Rheumatology, and Rehabilitation, Warsaw, Poland (approval protocol no: KBT-8/4/20016). All patients gave their written informed consent prior to enrolment.
Adipose tissue-derived mesenchymal stem cell isolation and culture
Specimens of subcutaneous abdominal fat were taken from the patients by 18 G needle biopsy. Tissue processing, ASC isolation and culture were performed as described previously [18]. Five human adipose-derived mesenchymal cell lines (Lonza Group, Lonza Walkersville Inc., MD, USA) were used as a control.
All experiments were performed using ASCs at 3–5 passages. Adipose tissue-derived mesenchymal stem cells were cultured in complete culture medium composed of DMEM/F12 (PAN Biotech United Kingdom Ltd., Wimborne, UK), 10% fetal calf serum (FCS) (Biochrom, Berlin, Germany), 200 U/ml penicillin, 200 µg/ml streptomycin (Polfa Tarchomin S.A., Warsaw, Poland) and 5 µg/ml Plasmocin (InvivoGen, San Diego, CA, USA).
Both untreated and cytokine pre-stimulated ASCs were applied. For pre-stimulation, ASCs were cultured for 24 hours with human recombinant tumour necrosis factor (TNF) and interferon gamma (IFN-γ) (both from R&D Systems, Minneapolis, MN, USA; each applied at 10 ng/ml).
Co-culture of adipose tissue-derived mesenchymal stem cells with purified allogeneic CD4+ T-cells
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from healthy male honorary blood donors (< 60 years old), according to the routinely applied procedure using Ficoll-Paque (GE Healthcare, Uppsala, Sweden). The CD3+CD4+ cells were isolated from PBMCs using the EasySep Human CD4+ T-Cell Isolation Kit (Stemcell Technologies, Vancouver, Canada).
After isolation, CD4+ T-cells (1.2 × 106/well/2 ml of medium) were seeded either directly (contacting cultures) or on 0.4 mm pore size Transwell filters (MD24 with carrier for inserts 0.4 MY, Thermo Fisher Scientific, Massachusetts, MA, USA) (non-contacting co-culture) into 24-well plates with adherent ASCs (5 × 104/well/2 ml of medium), then T-cells were activated with Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific, Massachusetts, MA, USA).
After 5 days of co-culture, CD4+ T-cells and culture supernatants were harvested for further analysis, i.e. flow cytometry to identify proliferating and non-proliferating cells, and the measurement of concentrations of kynurenines, PGE-2, IL-10, and IL-1Ra, respectively. The CD4+ T-cells cultured separately were used as a control.
Proliferation assay
For proliferation assay, purified CD3+CD4+ T lymphocytes were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) or cell trace violet (CTV) (Thermo Fisher Scientific, Massachusetts, MA, USA), then co-cultured with ASCs as described above.
Cells harvested from cultures were analysed by flow cytometry to identify proliferating and non-proliferating cells. To characterize the cellular proliferation response, the percentage of proliferating cells, proliferation index (PI – number of division per proliferating cell), and replication index (RI – fold expansion) were calculated as described elsewhere [19].
Measurement of soluble factor concentrations in culture supernatants
The concentration of PGE-2 was measured using the Parameter Kit (R&D Systems, Minneapolis, MN). Kynurenine concentration was measured spectrophotometrically as described previously [16].
To this aim, culture supernatants were mixed with 30% trichloroacetic acid at a 2 : 1 ratio and incubated for 30 min at 5°C, then centrifuged at 10 000 × γ for 5 min and finally diluted at a 1 : 1 ratio in Ehrlich’s reagent (100 mg of p-dimethyl benzaldehyde and 5 ml of glacial acetic acid; Sigma-Aldrich, St. Louis, MO, USA).
The optical density of the samples was measured at a wavelength of 490 nm. L-kynurenine (Sigma-Aldrich, St. Louis, MO, USA) diluted in culture medium was used to prepare the standard curve. The concentrations of cytokines were measured by specific enzyme-linked immunosorbent assays (ELISAs), i.e. IL-1Ra using ELISA DuoSet Kits from R&D Systems, and IL-10 using human IL-10 ELISA (cat. no. 88-7104-88) from Invitrogen (Vienna, Austria). All measurements were taken in duplicate.
Data analyses
Data were analysed using GraphPad Prism software version 7. The Shapiro-Wilk test was used as a normality test. The Wilcoxon test was applied to compare the secretion of soluble factors by resting and activated CD4+ T-cells.
One-way analysis of variance (ANOVA) with repeated measures and the post-hoc Tukey test were used to assess the effect of untreated and TNF/IFN-γ (TI)-treated ASCs (ASCsTI) on target cells, as well as to compare contacting versus (vs) non-contacting co-cultures.
The Mann-Whitney test was applied to analyse differences between effects exerted by ASCs of healthy donors versus ASCs of ankylosing spondylitis patients. Parametric (Pearson’s linear) and non-parametric (Spearman’s rank) correlation tests were used to assess associations between analysed parameters. Probability values less than 0.05 were considered significant.
Results
Patients
The patient cohort was heterogenous with respect to demographic and clinical data (Table I). All patients were HLA-B27 positive, 50% of them presented ocular symptoms (iritis), and none of them had peripheral arthritis. They were mostly treated with non-steroid anti-inflammatory drugs (NSAIDs), while application of non-biologic disease-modifying anti-rheumatic drugs (DMARDs) and glucocorticosteroids was less frequent.
Inhibition of T-cell proliferation by adipose tissue-derived mesenchymal stem cells
In control, separately cultured, purified CD4+ T-cells activated via the CD3/CD28 pathway the majority of cells proliferated (mean ±SEM = 63.9 ±3.4%).
In the presence of both untreated and TI-stimulated ASCs, the number of proliferating T-cells decreased significantly. The number of proliferating CD4+ T-cells in T-ASCs co-cultures was inhibited in the presence of HD/ASCs and HD/ASCsTI by 50.7 ±5.9% and 63.8 ±5.1%, respectively, while AS/ASCs and AS/ASCsTI reduced it by 42.2 ±6.8% and 49.2 ±7.6%, respectively (mean ±SEM; data not shown).
However, as shown in Figure 1A–C, there were no significant differences between inhibitory effects exerted by HD/ASCs and AS/ASCs on T-cell proliferation response, i.e. the percentage of proliferating cells, the proliferation, and replication indices.
Fig. 1
Inhibition of T-cell proliferation by adipose-derived stem cells of healthy donors and ankylosing spondylitis patients. T helper (CD3+CD4+) lymphocytes, isolated from peripheral blood of 13 (A–C) or 3 (C–F) healthy donors, were stimulated with anti-CD3/anti-CD28 antibodies and cultured alone (T) or co-cultured for 5 days with either untreated or TNF+IFN-γ (TI)-stimulated ASCs from 5 HD (HD/ASCs) or 10 AS patients (AS/ASCs). The cell co-cultures were performed in conditions allowing direct cell contact (A–C) and in a transwell system (D–F, as indicated). Proliferation of CD4+ T-cells was analysed by flow cytometry. Data are the results of the indicated number of experiments (n), performed using random combination of ASCs and T-cells. Upper panels (A–C) – lines between points identify cultures containing the same combination of ASCs and T-cells. Lower panels (D–F) – results are expressed as the median (horizontal line) with interquartile range (IQR, box), lower and upper whiskers (data within 3/2 x IQR) and outliers (points) (Tukey’s box). Values: *p = 0.05–0.01; **p = 0.01–0.001, ***p = 0.001–0.0001 for intra-group comparisons. The inter-group (HD vs. AS and contact vs. transwell co-cultures) differences were statistically insignificant.
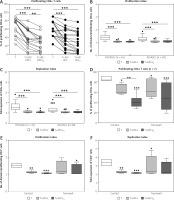
Moreover, untreated and TI-treated AS/ASCs reduced T-cell proliferation to a similar extent, while TI-treated HD/ASCs were more potent than untreated HD/ASCs in diminishing the number of proliferating cells (Fig. 1A), but the reduction of PI and RI values by these cells was comparable (Figs. 1B and 1C).
Contribution of cell-to-cell contact and soluble factors to anti-proliferative effect of adipose tissue-derived mesenchymal stem cells
Results of co-culture of untreated and TI-treated HD/ASCs with CD4+ T lymphocytes in the conditions allowing or preventing (transwell) direct cell-to-cell contact showed that inhibition of T-cell proliferation did not differ significantly between these culture systems (Figs. 1D–F), although in transwell cultures more scattered data were obtained.
These results point to secretory factors as critical mediators of ASC-triggered anti-proliferative effects. Therefore, we further investigated the production of several soluble factors in ASC/CD4+ T-cell co-cultures.
Production of soluble factors in co-cultures of CD4+ T-cells with adipose tissue-derived mesenchymal stem cells
Untreated and activated CD4+ T-cells produced small quantities of kynurenines (mean ±SEM = 0.004 ±0.001 vs. 0.05 ±0.02 mmol/ml, n = 7, p = 0.02 for untreated vs. anti-CD3/CD28-stimulated T-cells) and PGE-2 (0 vs. 815 ±531 pg/ml, n = 15, p = 0.09 for untreated vs. anti-CD3/CD28-stimulated T-cells) (data not shown). In the co-cultures of activated CD4+ T-cells with HD/ASCs and AS/ASCs significant increases of kynurenines and PGE-2 production were observed and untreated and TI-treated ASCs exerted similar enhancing effects (Figs. 2A and 2B). Importantly, both untreated and TI-treated AS/ASCs induced higher secretion of these factors, especially PGE-2, than HD/ASCs (Figs. 2A and 2B).
Fig. 2
Concentrations of soluble factors in the co-cultures of T-cells with adipose tissue-derived mesenchymal stem cells. Cells were prepared and co-cultured as described in Figure 1. CD4+ T-cells were isolated from peripheral blood of 10–17 healthy blood donors. Five HD/ASCs lines and AS/ASCs obtained from 6–8 patients were used. The concentrations of indicated soluble factors in culture supernatants were measured as described in the material and methods section. Data are the results of the indicated number of experiments (n), performed using random combination of ASCs and T-cells, and are shown as Tukey’s boxes. Values: ***p = 0.001–0.0001 for intra-group (T vs. T+ASCs) comparisons; #p = 0.05–0.01, ##p = 0.01–0.001 for inter-group (HD vs. AS) comparison.
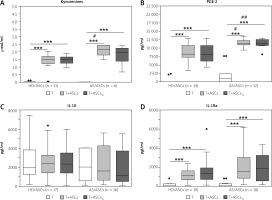
In separate cultures, CD4+ T-cells activated via the CD3/CD28 pathway produced significantly more IL-10 and IL-1Ra than resting cells. The concentrations of these cytokines in the culture supernatants of resting versus activated T-cells (mean ±SEM, n = 7) were as follows: 0 vs. 1906 ±165 pg/ml, p = 0.02, for IL-10 and 70 ±33 vs. 216 ±33 pg/ml, p = 0.02, for IL-1Ra. In the co-cultures of activated CD4+ T-cells with untreated and TI-treated HD/ASCs and AS/ASCs no significant changes were observed in the concentrations of IL-10 (Fig. 2C), while a significant and comparable increase of IL-1Ra levels was found in these conditions (Fig. 2D).
Dependence of kynurenines, PGE-2, IL-10, and IL-1Ra production on cell-cell contact and soluble factors
In the cell-to-cell contact and transwell co-cultures of CD4+ T-cells with HD/ASCs the concentrations of PGE-2 were similar (Fig. 3B), whereas kynurenines levels were higher in transwell than cell contacting co-cultures, especially in those containing TI-treated HD/ASCs (Fig. 3A). By contrast, the concentrations of IL-10 (Fig. 3C), and to a lesser extent also IL-1Ra, (Fig. 3D) were significantly lower in the transwell system than in cell contacting co-cultures.
Fig. 3
Comparison of soluble factor production in contact and transwell co-cultures. Cells were prepared and co-cultured as described in Figure 1. Five HD/ASCs lines and CD4+ T-cells from peripheral blood of 5–7 healthy blood donors were used. The concentrations of indicated soluble factors in culture supernatants were measured as described in the material and methods section. Data are the results of the indicated number of experiments (n), performed using random combination of ASCs and T-cells, and are shown as Tukey’s boxes. Values: *p = 0.05–0.01, **p = 0.01–0.001, ***p = 0.001–0.0001 for intra-group (T vs. T+ASCs) comparison; #p = 0.05–0.01, ##p = 0.01–0.001, ###p = 0.001–0.0001 for comparison of contact vs. transwell cultures.
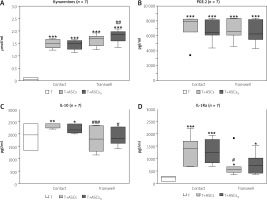
Thus, these results indicate that in the co-cultures of purified CD4+ T-cells with HD/ASCs up-regulation of kynurenines and PGE-2 release is dependent mostly on soluble factors, while cell-to-cell contact is important to raise IL-1Ra and to maintain IL-10 levels, respectively.
Association of soluble factor secretion with anti-proliferative effect of adipose tissue-derived mesenchymal stem cells
As shown in Table II, in the co-cultures containing untreated HD/ASCs or AS/ASCs the proliferative response of T-cells inversely correlated with the concentration of PGE-2 or kynurenines and IL-10, respectively. The association was more evident in the presence of TI-treated HD/ASC and AS/ASCs (Table II, Fig. 4).
Table II
Correlation between soluble factor concentrations and T-cell proliferation in co-cultures with adipose tissue-derived mesenchymal stem cells
[i] ASCs – adipose tissue-derived mesenchymal stem cells, AS/ASCs – ASCs from ankylosing spondylitis patients, HD/ASCs – ASCs from healthy donors, n – number of performed experiments, TI-treated – ASCs pre-stimulated with TNF+IFN-γ, R – Pearson’s correlation coefficient, Rs – Spearman’s rank correlation coefficient; p-values < 0.05 and < 0.1 are marked in bold and underlined font, respectively.
Fig. 4
Correlation between concentrations of soluble factors and T-cell proliferative response. Cell co-culture, the evaluation of T-cell proliferation, and measurement of indicated soluble factor concentrations were performed as described in the materials and methods section. Pearson’s (R) or Spearman’s rank (Rs) correlation coefficient and p-values are shown. Other explanations as in Figure 1.

There was a moderate to strong negative correlation between the replication index on the one hand, and the concentrations of kynurenines, PGE-2, and IL-10, but not IL-1Ra, on the other hand (Fig. 4).
However, in the presence of HD/ASCsTI, the association between RI values and kynurenines levels did not reach statistical significance (R = –0.652, p = 0.079), and PGE-2 concentration inversely correlated with the percentage of proliferating T-cells but not with RI (Fig. 4, Table II).
In the case of AS/ASCsTI-containing co-cultures an inverse correlation was also found between the proliferation index and the levels of soluble factors, such as kynurenines, PGE-2, and IL-10 (Table II).
Discussion
The anti-proliferative effects of MSCs on T-cells have been demonstrated in vitro and in vivo [10, 11, 20, 21]. It is well documented that to exert these effects MSCs act directly [22–24] or indirectly via engagement of intermediary cells [25, 26].
Unfortunately, it is unknown whether ASCs of AS patients have these capabilities preserved. Considering the contribution of T-cell activation and expansion to AS pathology [4–9], and taking into account possible therapeutic application of AS/ASCs [27], functional characterisation of these cells is urgently required.
We have previously found that in comparison with HD/ASCs, separately cultured untreated and TI-treated AS/ASCs secrete less kynurenines, more IL-1Ra, but comparable amounts of PGE-2 [16]. In addition, we have recently reported that AS/ASCs fail to downregulate a CD25 activation marker (IL-2 receptor a chain) on co-cultured CD4+ T-cells [28].
Despite these abnormalities, present results show that both HD/ASCs and AS/ASCs inhibit the proliferative response of purified allogeneic CD4+ T-cells with similar potency, reducing the number of proliferating cells, the number of divisions of these cells (PI), and their fold expansion over the culture time (RI) (Figs. 1A–C).
Thus, our results evidence normal anti-proliferative effects of AS/ASCs exerted on T helper (CD4+) cells in a direct manner. The immunomodulatory effects of MSCs are mediated by both direct cell-to-cell interactions and soluble factors [10, 11, 29, 30]. Consistently with the reports of others [21, 31, 32], we have confirmed that the anti-proliferative effect of HD/ASCs is mediated mostly by soluble factors (Figs. 1D–F).
Because several soluble mediators, including kynurenines, PGE-2, and IL-10, have been reported to mediate the anti-proliferative effect of MSCs [31, 33], we also measured concentrations of these factors in co-culture supernatants. As shown in Figure 2, co-culture of CD4+ T-cells with ASCs significantly up-regulated the levels of kynurenines, PGE-2, and IL-1Ra, and the release of the first two factors was even higher in the co-cultures containing ASCs from AS patients than healthy donors.
By contrast, the concentration of IL-10 did not change significantly (Fig. 2C). Because separately cultured ASCs [16] and activated CD4+ T-cells (see Results) produce small amounts of kynurenines, PGE-2, and IL-1Ra, it is clear that the significant, synergistic increase of production of these factors results from interactions of co-cultured ASCs and activated CD4+ T-cells.
To verify whether these cell interactions depend on direct cell-to-cell contact or are mediated by soluble factors, the concentrations of kynurenines, PGE-2, IL-10, and IL-1Ra were also measured in contacting and non-contacting (transwell) co-culture systems (Fig. 3).
The obtained results show that HD/ASCs act by two distinct mechanisms. The first of these, which does not require cell-to-cell contact, up-regulates the release of PGE-2 and kynurenines (Figs. 3B and 3A, respectively). The second mechanism, which is contact dependent, controls anti-inflammatory cytokine levels, i.e. mediates IL-1Ra increase and maintenance of IL-10 levels.
Thus, our results regarding ASCs supplement the observations of others, showing the prominence of soluble factors in controlling kynurenine and PGE-2 production by various types of MSCs [31, 33], and cell contact-dependent up-regulation of IL-10 gene expression in BM-MSCs [34].
Among tested factors, the kynurenine pathway and PGE-2 were reported to be critically involved in mediating anti-proliferative effects of various types of MSCs on T-cells, especially in humans [20, 31, 32, 35, 36].
In the first pathway, the essential amino acid tryptophan is metabolised by indoleamine 2,3-dioxygense (IDO) to kynurenines, and both local tryptophan deprivation and kynurenine accumulation result in the inhibition of T-cell proliferation [32, 37, 38]. Similarly, PGE-2 triggers cell cycle arrest of T-cells [25, 32, 36].
In addition, IL-10 can directly inhibit activation and proliferation of T-cells by altering the CD28 co-stimulation pathway [39]. Consistently with known anti-proliferative capabilities of the above factors, the present results show a significant, moderate to strong, inverse correlation between tested parameters of the T-cell proliferative response and the concentrations of kynurenines, PGE-2 and IL-10, especially in the co-cultures containing TI-pre-stimulated ASCs (Table II, Fig. 4). The priming of MSCs with pro-inflammatory cytokines, e.g. with TNF and/or IFN-g, a procedure named “licensing”, is known to enhance the immunosuppressive functions of these cells, including inhibition of T-cell proliferation [40].
Importantly, we observed that the association of the levels of soluble factors with T-cell proliferation was more evident in co-cultures containing TI-pre-stimulated ASCs of AS patients than healthy donors (Table II).
One possible explanation is significantly higher production of kynurenines and PGE-2 in the co-cultures of CD4+ T-cells with AS/ASCs than HD/ASCs (Figs. 2A and 2B). These observations suggest that AS/ASCs resemble, to a certain extent, in vivo “licensed” cells residing in chronically inflamed tissue.
As for the relationship between the proliferative response and IL-10, whose concentration was not affected by ASC presence, and was similar in co-cultures containing AS/ASCs and HD/ASCs, it is likely that the level of IL-10 receptor (IL-10R) expression on T-cells is important. It is known that the level of this cytokine receptor expression is closely dependent on T-cell activation, being very low on naïve while high on activated/memory cells, and thus regulates responsiveness of T-cells to IL-10 [41].
However, whether ASCs modulate IL-10R expression requires further investigations. By contrast to kynurenines, PGE-2, and IL-10, we did not observe any association of the concentration of IL-1Ra with the T-cell proliferative response (Table II, Fig. 4). This is consistent with the biological activity of IL-1Ra, which counteracts the pro-inflammatory effects of IL-1 and is a crucial mediator of MSCs-triggered immunosuppression of macrophages and B-cells, but does not affect CD4+ T-cell proliferation [42].
Conclusions
AS/ASCs similar to HD/ASCs also exert a direct effective anti-proliferative impact on CD4+ T-cells, acting via soluble factors that are released in cell contact-dependent (IL-10) and independent (kynurenines, PGE-2) pathways.
The results presented indicate that AS/ASC could be a potentially useful target for therapeutic applications.



