Introduction
Fibromyalgia (FM) is characterized by generalized and continuous musculoskeletal pain as a leading symptom. Pain is considered chronic when it persists for at least three months [1, 2].
The pain can be described as a form of chronic widespread pain (CWP). In accordance with the definition of the International Association for the Study of Pain (IASP), CWP is “pain in at least 4 of 5 body regions (in at least 3 or 4 body quadrants)” accompanied by functional symptoms [2].
Functional symptoms include fatigue, insomnia and/or insomnolence, cognitive impairment, mood disorders, and stiffness of the joints and muscles. These symptoms have been present at a similar level for at least 3 months and are not better accounted for by any other diagnosis [3, 4].
According to previous studies, altered central sensitization was postulated to play the main role in FM pathogenesis, but strict mechanisms have not yet been fully elucidated [3, 5–8]. The nature of the FM is controversial [9–11].
Fibromyalgia is a multifactorial disorder with an unknown underlying pathomechanism. Alternatively, FM is defined as a syndrome with a low pain threshold as the main symptom. A broad spectrum of factors is postulated to underlie the etiology, including genetic, neurogenic, immunogenic, and psychogenic ones [12].
Additionally, overlapping syndromes and disorders coexist with FM with high frequency [13–17]. However, they are similar enough to make differential diagnosis difficult [4, 18, 19].
Therefore, the diagnosis and treatment of FM are challenging for clinicians. Fibromyalgia as a subject concerning anesthesiologists was debated in 2009 and 2011 [20, 21]. Since then, significant advances have been made in understanding the pathophysiology of FM. In addition, a novel approach to diagnosis was made with the constant evolution of diagnostic criteria. Evidence-based recommendations for treatment have been proposed and evaluated.
Patients with FM constitute a potentially problematic population for perioperative care. Pain hypersensitivity, a characteristic feature of FM, is a diagnostic and therapeutic challenge in patients exposed to the acute pain stress associated with surgery [22]. The complex and not yet resolved abnormalities of nociception and pain perception processing in FM represent difficulties in diagnosis and treatment in the perioperative period. Moreover, controversies have arisen during discussions on FM etiology [23–25].
The primary research interests of these patients appear to be perioperative management optimization and individualized care. The main challenge is to identify research directions that will implement these goals.
The aim of this review is to present an update on FM assessment with special emphasis on pathogenetic, diagnostic, and therapeutic issues. This review focuses on the clinical usefulness and practical aspects of new research data in the evaluation of FM patients in perioperative care.
In addition, this review is a proposal for discussion for anesthesiologists and rheumatologists to improve the quality of perioperative care in FM patients and establish directions for cooperation.
Material and methods
The literature search included PubMed/Medline, Web of Science, Directory of Open Access Journals (DOAJ), and Cochrane Library search engines up to 31.12.2022. A search for the key word “fibromyalgia” yielded a total of 40,231 results (PubMed/Medline – 13,617, Web of Science – 20,890, DOAJ – 2,089 and Cochrane Library – 3,635). Because the scope of the literature is too extensive, the review was narrowed down, according to the Boolean methodology, using the following keywords: “fibromyalgia”, “etiology”, “treatment”, ”perioperative management”, and “nociplastic pain”.
The most relevant types of articles were included (clinical trial, meta-analysis, randomized controlled trial, review and systematic review). The search yielded a total of 12,181 results (2,368 for “fibromyalgia” and “etiology”, 9,616 for “fibromyalgia” and “treatment”, 90 for “fibromyalgia” and “perioperative management”, and 107 for “fibromyalgia” and “nociplastic pain”). The removal of duplicates, publications of non-English origin and off-topic articles limited references to 753.
A thorough review of the area of interest was then manually conducted to search for articles that presented actual data. Moreover, the most relevant papers from the cited articles’ references were added. The literature search included only articles published in English. Finally, the authors included into analysis and discussion 85 articles.
Results of searching
Prevalence
The prevalence of FM is estimated to be between 2% and 8% in the general population and increases with comorbidity of specific disorders [4]. The frequency of FM occurrence varies according to sex, with a female predominance.
The prevalence rate in the male population differs according to the methodology of the studies. The perception of FM as predominant (≥ 90%) in the population of women is not supported by data from unbiased studies [26].
Wolfe et al. [26] postulated that bias occurred in studies on FM prevalence by underestimating men and overestimating women. In an article from 2017, Marques et al. [27] presented the prevalence of FM in different countries as ranging between 0.2% and 8.8%. The FM prevalence rate in women took values between 2.4% and 6.8%. The rate of FM in urban areas varies between 0.7% and 11.4%, and in rural areas between 0.1% and 5.2% [27].
In a systematic review of FM epidemiology, Cabo-Meseguer et al. [28] reported a data sheet of FM prevalence worldwide. The mean prevalence of FM worldwide is estimated to be 2.1%, with 3.43% in women and 0.95% in men, with a proportion of 4 : 1.
Heidari et al. [29] performed a meta-analysis of the prevalence of FM. The results showed the prevalence of 1.78% in the general population, with noticeable predominance of women. A meta-analysis based on the subgroups showed prevalence of FM of 15.2% in patients referred to rheumatology clinics, 6.3% in hemodialysis patients, 12.9% among patients with irritable bowel syndrome (IBS) and 14.8% in patients with type 2 diabetes mellitus. Moreover, in the Behçet syndrome group, 80% of the patients developed FM [29].
Habib et al. [30] summarized diagnoses of FM in patients attending the rheumatology clinic. Fibromyalgia was diagnosed using the criteria of ACR 2010 and revealed a frequency of 42% as the primary diagnosis. The mean age was 38.9 ±–12.2 (range: 18–72), and the female : male ratio was approximately 4:1 [30].
In an observational case-control study, Coloma et al. [31] reported the incidence of FM prevalence in patients with deep infiltrating endometriosis (DIE) in comparison with superficial and ovarian endometriosis (non-DIE) and healthy subjects (C). Fibromyalgia was assessed using the London Fibromyalgia Epidemiological Study Screening Questionnaire (LFESSQ).
The estimated FM prevalence was higher in the DIE group than in the other two groups [31]. The risk of FM increases with coexisting disorders, especially when connective tissue diseases are diagnosed, as was shown in the articles. Fibromyalgia can be diagnosed in each age group [10].
In a systematic review and meta-analysis, Mansfield et al. [32] reported an increase in CWP between 40 and 50 years of age. In older age groups, either a continually increasing prevalence or plateauing of prevalence was observed. Susceptibility in older individuals is declining [32]. Aggravation of symptoms in the age 40–50 female population could be linked to a higher frequency of seeking medical attention.
Classification
The diagnosis of FM has been challenging for clinicians for several years. Due to the unrecognized exact pathomechanism and commonly occurring comorbidities, almost 75% of cases are underdiagnosed. However, overdiagnosis and misdiagnosis occur frequently [18]. One of FM’s leading symptoms – chronic widespread pain (CWP) – can be diagnosed as a standalone disease entity, but it usually leads to a diagnosis of FM. Diagnostic criteria require the presence of associated symptoms (sleep disturbance and/or fatigue).
Previously, the ICD-10 classified FM with code M79.7 as a “disease of the musculoskeletal system and connective tissue” in the group of “Other and unspecified soft tissue disorders, not elsewhere classified”. In accordance with the IASP criteria for ICD-11, the WHO (version 09-2020) classified FM as chronic primary widespread pain (code MG30.01).
Chronic widespread pain is described as “diffuse pain in at least 4 of 5 body regions and is associated with significant emotional distress (anxiety, anger/frustration or depressed mood) or functional disability (interference in daily life activities and reduced participation in social roles)”.
Chronic widespread pain is multifactorial. Multiple factors (biological, psychological, and social) contribute to the severity of the pain syndrome. In accordance with the IASP criteria, the diagnosis of CWP is appropriate when the pain is not directly attributable to regional nociceptive processes, and there are features consistent with nociplastic pain, as well as identified psychological and social contributors [2].
Nociplastic pain is a relatively new term introduced by the IASP in 2017 to classify pain syndromes of undetermined etiology [25, 33, 34]. This type of pain differs from nociceptive pain (caused by ongoing inflammation and/or tissue damage) and neuropathic pain (caused by nerve damage) [35].
Most likely, nociplastic pain is caused by increased nociceptive processes in the CNS. Disturbances in pain processing and altered pain modulation probably play a significant role in its development. Nociplastic pain is multifocal and often accompanied by other symptoms of central origin, such as fatigue, sleep disturbances, memory loss, and mood disorders [33–35] The above characteristics were well suited to FM.
Diagnostic criteria for FM were evaluated at the end of the last century and resulted in consecutive American College of Rheumatology (ACR) diagnostic criteria of 1990 and 2010 with revision in 2011 and 2016 [36–39].
In 2018, the ACTTION-APS Pain Taxonomy established an international FM working group. The authors of the project invited clinicians and researchers specializing in FM. The main goal of this initiative is to generate core diagnostic criteria for FM and apply the multidimensional diagnostic framework adopted by AAPT to FM [40]. The diagnostic criteria for ACR and AAPT are listed in Table I.
Table I
ACR/AAPT classification criteria for fibromyalgia. Source: Author’s own elaboration and based on references [36–41]
[i] ACR – American College of Rheumatology, ACTTION – Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations, APPT – ACTTION-APS Pain Taxonomy, Opportunities and Networks, APS – American Pain Society, FSS – Fibromyalgia Severity, Score, MSP – multisite pain, SSS – Symptom Severity Score, WPI – Widespread Pain Index.
Diagnostic criteria for fibromyalgia became problematic at the turn of the 20th century [41]. The first mention of fibromyalgia as a term of “muscular rheumatism” originates from “Liber de Rheumatismo et Pleuritide Dorsali” by Guillaume de Baillou in 1642 [42].
From the beginning of the twentieth century with the works of Gowers [43] and Stockman [44], a new era of research in fibromyalgia began and reached a peak at the new millennium [45–47]. However, the term “fibromyalgia” was introduced by Hench in 1976 [48].
The works of Yunus et al. [49] in the 1980s began the systematic approach to FM by describing prototypic diagnostic criteria. In 1987, FM was accepted by the American Medical Association as a disease. Goldenberg wrote the first article on FM [50]. In 1990, the American College of Rheumatology (ACR) established the first ARC FM diagnostic criteria [36]. The ARC diagnostic criteria were revisited in 2010 [37] and evaluated in 2011 [38] and 2016 [39] (Table I).
The Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks (ACTTION) public-private partnership with the U.S. Food and Drug Administration (FDA) and the American Pain Society (APS) initiated the ACTTIONAPS Pain Taxonomy (AAPT) to develop a consistent diagnostic system for chronic pain disorders [40].
The auspices of the AAPT international FM working group were established to generate the core diagnostic criteria for FM. The main objective of this study was to create a multidimensional diagnostic framework for FM [40]. The process for developing the AAPT criteria and dimensions included literature reviews and synthesis, and consensus discussions. Moreover, analyses of data from large population-based studies conducted in the United Kingdom were performed [40].
The idea of AAPT diagnostic criteria is to divide the diagnostic process of FM into five dimensions, allowing for a synthetic approach to FM. The concept of the AAPT-FM workgroup was to establish a revised diagnosis of FM and identify the risk factors, course, prognosis, and pathophysiology of FM [40].
The workgroup determined that the core diagnostic criteria (dimension 1) for FM are pain of at least 3 months duration occurring in at least six body sites (usage of the nine-site manikin) and defined as multisite pain (MSP).
Pain is accompanied by fatigue (physical or mental) or sleep disturbances judged to be of at least moderate severity by a clinician. Other common features (dimension 2) include tenderness (widespread heightened sensitivity to pressure), executive functioning deficits (disorganized/slow thinking, difficulty concentrating, forgetfulness), and sensory intolerance (heightened sensitivity to light, sounds, odors, or cold). Common comorbidities associated with FM (dimension 3) can be divided into several groups, including somatic pain disorders, psychiatric conditions, sleep disorders, rheumatic diseases, and others [40].
Future studies will assess the criteria for feasibility, reliability, and validity. Revisions of the dimensions will also be required as research advances in FM understanding. The AAPT Diagnostic Criteria for FM (AAPT-FM) met with controversial acceptance [51].
The 2011/2016 ACR and AAPT criteria were compared by Salafii et al. [52]. In the conclusion they noted considerable agreement in the diagnosis of FM, although AAPT criteria performed least well [52]. The significant advantage of AAPT diagnostic criteria is the new look at the diagnostic tools in FM and invitation for further discussion on improvement.
Etiology
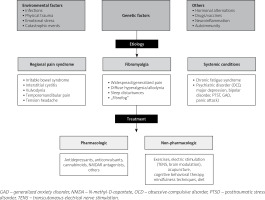
The etiology of FM has not yet been determined. In the literature, triggers have been presented, including infections, emotional stress, and physical trauma [3, 4]. Several hypotheses on the pathogenesis of FM have been proposed in recent years. The main aspects of the research included genetic susceptibility, environmental factors, neuromodulation, autoimmunity, and neuroinflammation.
Genetic susceptibility has been reported in familial studies of patients with FM [53]. Researchers have found evidence elucidating the genetic mechanisms in FM. Epigenetic studies have focused on genetic variants and inheritance mechanisms in pain-related gene studies, DNA methylation, miRNA profiles as potential FM biomarkers, and hypothetical histone modifications [54, 55].
In a comprehensive review, D’Angelli et al. [54] presented results of genome studies that could be implicated in understanding the pathogenesis, potential biomarkers, and new treatments for FM. Potential genes associated with FM include the serotonin transporter gene (SLC64A4), transient receptor potential vanilloid channel 2 gene (TRPV2), myelin transcription factor 1 like gene (MYT1L), and neurexin 3 gene (NRXN3). Differences in DNA methylation and altered miRNA profiles were found in patients with FM compared to healthy controls [54].
In bioinformatic analysis performed by Qiu et al. [55], differentially expressed genes (DEGs) and mi-RNAs (DEMs) were found between FM and normal blood samples. The results of this study show that CD38, GATM, HDC, and FOS are candidate genes for FM.
These genes are significantly associated with musculoskeletal diseases, mental disorders, and immune system diseases. Studies have also observed a partial overlap between metformin therapy-related genes and FM-related genes [55].
As described by Saleh et al. [56] transposable elements (TEs) are mobile DNA elements which replicate and insert themselves into different locations within the host genome. The development of nucleic acid sequencing technology has revealed that almost half of the human genome consists of TEs. Human endogenous retroviruses (HERVs) and long-interspersed nuclear element-1 (LINE-1) are the two main classes of retrotransposons (TEs).
Human endogenous retrovirus and LINE-1 insertions, through a “copy and paste” mechanism, have accumulated throughout evolution. The host genomes have simultaneously coevolved with TEs using various factors to suppress aberrant activity [56].
Human endogenous retroviruses have been associated with the pathogenesis of several autoimmune and neurologic diseases, including myalgic encephalomyelitis and chronic fatigue syndrome (ME/CFS) [57]. Ramírez-Morales et al. [17], in a meta-analysis from 2022, found a prominent clinical overlap between FM and myalgic encephalomyelitis (ME). The results were more pronounced with ACR 2016 fibromyalgia diagnostic criteria.
Ovejero et al. [57] observed over-expression of HERVs (H, K, and W types) in immune cells of FM patients, regardless of ME/CFS comorbidity. In addition, these patients presented increased levels of interferons (INF-β and INF-γ) but unchanged levels of tumor necrosis factor α (TNF-α). These findings may explain the widespread pain manifestations in FM [57].
In a comprehensive review, Grace et al. [58] presented neuroimmune signaling mechanisms in chronic pain. The main mechanism of chronic pain onset seems to be persistent activation of the nociceptive system in a malfunctioning manner.
The prevalence of chronic pain in adult populations is estimated to be up to 20% in developed countries. Post-surgical chronic pain (PSCP) occurs in 10% of patients undergoing surgery [5], and the prevalence of FM as mentioned above varies between 2 and 15% depending on the type of cohort evaluated [4, 27]. Based on these studies, the hypothesis of a mixed pathomechanism of chronic pain in FM was postulated in a similar manner to FM etiology.
Two main processes could be involved in chronic pain pathomechanism in FM: central and peripheral sensitization [4, 5, 7]. New hypotheses in nociception research postulate the presence of special intercellular crosstalk between neurons, glia, and immune cells. Nociceptive signaling transmission induces hyperalgesic priming of primary afferent neurons. The response to noxious stimulation from primary injury is multicellular.
The three main responding systems are the neuronal network, glial tissue, and immune system [59]. Some researchers have classified glia as an integral part of the immune system [60]. Persistent immune activation with prolonged inflammation promotes chronification of nociception and transformation of acute pain to chronic pain [5]. These mechanisms may explain the origin of nociplastic pain.
O’Mahony et al. [61] in a systematic review outlined the differences in the peripheral blood cytokine profiles between FM patients and healthy controls. Proinflammatory cytokines may lead to nociceptive activation in FM. The FM signature includes both pro-inflammatory (TNF-α, interleukin-6, interleukin-8) and anti-inflammatory (interleukin-10) cytokines and chemokines (eotaxin) [61, 62].
The other aspect of interactions in cell cross-talk is memory. Both immune and nervous systems generate processes that can be defined as biochemical/structural memory. Sarzi-Puttini et al. [4] presented a very interesting hypothesis of the interplay between potential pathogenic mechanisms and nociplastic alterations in FM.
As mentioned above, the pain in FM can be considered as nociplastic pain [2, 23, 24]. Interplay between various mechanisms, including genetic predisposition, stress, peripheral (inflammatory), and central (cognitive–emotional) mechanisms lead to neuromorphological modifications and pain dysperception [60, 62].
A reciprocal etiopathogenic relationship might also occur between the central and peripheral nervous systems in both bottom-up and top-down fashion, provoking aggravation of symptoms in FM [4].
The multifactorial etiology of FM with a prominent role of biopsychosocial factors, evident familial susceptibility, and abnormalities in nociceptive processes with central and probably peripheral sensitization seems to present a great challenge for future studies.
Treatment
Treatment of FM should be comprehensive. The first step is to educate the patient about the nature of the disorder, treatment plan, and outcome prognosis. Fibromyalgia is regarded as a biopsychosocial disorder or syndrome, so the main directions of therapy should be focused on these aspects.
Genetic susceptibility and triggering psychosocial factors, such as stress, can aggravate symptoms and worsen outcomes. The reduction of anxiety should be a priority in FM therapy. Evidence-based therapy is based on a multimodal approach that includes all aspects of the FM. An individualized tailored approach is recommended, with a special focus on multidisciplinary therapy [63].
Nonpharmacological treatment of FM with strong recommendations from the European Alliance of Associations for Rheumatology (EULAR) is aerobic and strengthening training. Other nonpharmacologic therapies include cognitive behavioral therapies, multicomponent therapies, acupuncture, SPA therapy, and meditative movement therapies such as Qigong, Yoga, and Tai Chi, and mindfulness-based stress reduction therapies (MBSR) have weak recommendations [63].
Pharmacological treatment of FM includes several drugs approved by the FDA and EULAR. Drug treatments in FM must take into account the potential risk of adverse effects, including cognitive disturbances. The lowest effective doses should be administered.
Recommended drugs include pain modulators such as serotonin and noradrenaline reuptake inhibitors (SNRI): duloxetine and milnacipran; low doses of the tricyclic antidepressant agent amitriptyline; and the antiepileptic agent pregabalin.
Non-recommended drugs for FM include simple analgesics (acetaminophen, metamizole/dipyrone), nonsteroidal anti-inflammatory drugs, glucocorticosteroids, growth hormone, strong opioids, and sodium oxybate. Tramadol has an Ib recommendation from the EULAR [63]. Noteworthy are the works on the use of cannabinoids in the treatment of FM. However, valuable clinical trials are still pending [64, 65].
Perioperative assessment and management
The most recent publications regarding anesthesia care in patients with FM were published more than ten years ago [20, 21].
There have been some studies on FM patients undergoing different surgical procedures. Janda et al. [66] in a prospective study of patients undergoing hysterectomy after preoperative assessment of ACR2011 FM criteria observed that higher FM survey scores were significantly associated with worse preoperative pain characteristics.
This study showed an increase in pain scores, higher incidence of neuropathic pain, greater psychological stress, and increased perioperative opioid use in patients with higher FM survey scores [66]. Similar findings have been previously obtained in total knee and hip arthroplasty cohort studies [67].
In an observational cohort study by Ablin et al. [68] patients with FM scheduled for spine surgery were evaluated. In this study, a negative correlation was observed between the presurgical severity of FM symptoms and components of the postsurgical SF-36 questionnaires [68].
In a retrospective review of FM patients who had undergone lumbar spine fusion for degenerative lumbar pathology, Donnally 3rd et al. [69] noted a higher prevalence of perioperative complications compared to controls. In a retrospective cohort study, Sodhi et al. [70] assessed the risk of surgical complications after total knee arthroplasty (TKA) in FM patients. The analysis involved over 300,000 patients. The results showed that patients with FM have a greater risk of developing certain surgical complications after TKA [70].
These findings are in agreement with the conclusions presented by other researchers, suggesting that patients with FM, such as patients with rheumatoid arthritis (RA), may have a less favorable outcome following the surgical procedures and require a tailored perioperative anesthetic regimen to lower the risk of possible complications [71].
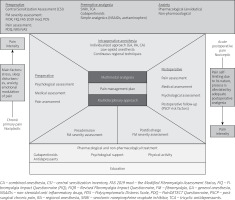
Multimodal analgesia has been defined as “use of more than one modality of pain control to achieve effective analgesia while reducing opioid-related side effects”. Multimodal and preemptive analgesia as part of enhanced recovery after surgery (ERAS) protocols shorten the recovery period after surgery, decrease postoperative morbidity, and reduce the cost of medical care [72]. Preoperative anesthetic evaluation included a meticulous history of coexisting diseases.
Unfortunately, a high number of FM cases are under- and misdiagnosed [18, 26]. This situation creates a need to establish a quick clinical assessment of FM patients before the preoperative anesthetic approach. In some studies, the painDETECT questionnaire, a popular tool for evaluating neuropathic pain, was used for FM patients [73, 74].
Another promising screening device is the central sensitization inventory (CSI) [75, 76]. Moreover, disease-specific multi-dimensional FM patients’ self-measurement tools (FIQR, FIQ, FAS19mod, FSC) also could be used in primary perioperative evaluation [11].
Further studies are needed to estimate the clinical values of these questionnaires in perioperative management of FM patients. Previous hypotheses on alterations of the endogenous opioid system in FM subjects have weak evidence, and further studies should be performed [77]. Although EULAR recommendations showed reluctance for opioid therapy in FM, postoperative analgesia was based on these drugs.
To avoid the possible risk of opioid overuse, three directions could be proposed: multimodal low-opioid anesthesia, partial or mixed opioid agonists for postoperative pain therapy, and continuous regional techniques.
All methods should be combined with multimodal preventive analgesia, comply with ERAS protocols, and be individualized for each patient. This proposal needs further validation in patients with FM. Low-opioid anesthesia can provide a possible prophylactic regimen for opioid-induced hyperalgesia in these patients [78].
Discussion
The prevalence of FM varies depending on social and environmental factors. The diagnosis of FM seems to depend on different diagnostic criteria, individual experience of the physician, and presentation of the patient. The proposal of IASP of new classification criteria could facilitate a reliable FM diagnosis and therefore help to provide effective treatments in the future [2].
Promising data from neuromolecular studies give hope for further clarification of the etiology and pathomechanism of FM [4, 5, 7, 8]. Unfortunately, complicated diagnostic criteria and the high degree of underdiagnosis and misdiagnosis of FM are challenging for clinicians [18].
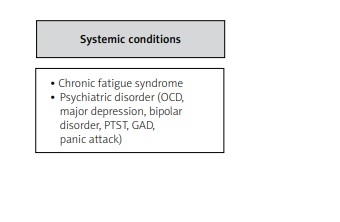
Other factors complicating the correct diagnosis are frequently overlapping syndromes [18, 40] (Table II).
Table II
Overlapping syndromes and frequent fibromyalgia comorbidities. Source: Author’s own elaboration and based on references [4, 40, 41]
For this reason, the diagnosis of FM should be made following careful exclusion of other conditions causing similar symptoms and focused on the possibility of comorbidities, especially from the spectrum of connective tissue diseases. Another important issue that should be considered is the possible gender bias in the diagnosis of FM in an underdiagnosed male population [26].
The core symptoms of FM include widespread chronic musculoskeletal pain, sleep disturbances, chronic fatigue, cognitive impairment, and mood disorders. The diagnostic criteria did not differentiate between the chronology of onset of these symptoms and presented all of them in a cohort manner. Further studies on the chronology of FM symptoms need to be performed.
Central sensitization seems to play a major role in both the etiology of FM and the chronification of pain in the postoperative period [5]. Chronic pain is a crucial problem in postoperative care. Personalized pain medicine, the idea of Bruehl et al. [79], should be considered in every patient diagnosed with and treated for FM. A synthesis of the FM approach and main directions in perioperative management based on the discussed literature are shown in Figures 1 and 2 [4, 11, 13, 20, 33].
In the article published by Sarzi-Puttini et al. [11], the authors synthesized the main directions for the future management of FM patients by highlighting the multimodal approach. Moreover, in the most recently published articles the authors have presented yearly updates of new data on FM [80–85].
Conclusions
The main issue in the diagnosis and management of FM is to focus on the evaluation of strict diagnostic criteria to minimize under- and overdiagnosis. Multimodal therapy with an individualized approach seems to be the optimal solution for FM in the perioperative period.
Further research should be performed to focus on global prevalence, etiology, pathomechanism, and their implications for further treatment.
Interdisciplinary research with special interest in pain management, including perioperative medicine, seems to be the main theme for the future.