Introduction
The term amyloidosis encompasses a group of heterogeneous, either acquired or congenital disorders characterized by accumulation of extracellular deposits of abnormal, insoluble proteins in various organs, which leads to functional impairment. These deposits contain b polypeptide chains that stain well with Congo red dye and show characteristic birefringence in polarized light [1]. Novel therapies are being introduced to inhibit deposit accumulation [2]. It is crucial to diagnose amyloidosis early, when deposits are minimal and there is only slight tissue damage. Such early diagnosis affords a chance to stem disease progression and improve prognosis [3]. The diagnosis is established based on biopsy and subsequent histological examination confirming the presence of amyloid deposits in tissues. Only in transthyretin-amyloidosis (ATTR) has the diagnosis recently become noninvasive with the use of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scintigraphy [4]. The widely adopted standard in histochemical assessments for amyloidosis is the use of the classic alkaline Congo red (ACR) staining method described by Puchtler [5] or its modification [6, 7]. Amyloid deposits stain red and show apple-green birefringence in polarized light [8]. Detecting red/orange fluorescence of amyloid deposits under fluorescent microscopy may also aid in the diagnosis [9]. Laboratory practice has shown varied staining intensity, possibly depending on the type of amyloid and tissue and the duration of fixation [10, 11]. Weakly stained minimal deposits may be missed and remain undetected. There is a need for a simple, reliable, and inexpensive staining technique, which would help avoid false negative results and might become an effective screening test readily available at clinical diagnostic laboratories. This is why we conducted a comparison of two tissue staining methods, ACR and phenol Congo red (PHCR), using tissue specimens from patients with clinically suspected amyloidosis [12]. This paper presents the results of the comparison in terms of amyloid detectability with the two staining methods.
Material and methods
The study was conducted using archived tissue samples sent to the Pathology Laboratory of the National Institute of Geriatrics, Rheumatology and Rehabilitation in Warsaw between the years 2011 and 2020. The samples, which were fixed in buffered formalin and some of them were embedded in paraffin blocks, had been obtained from 425 patients with clinically suspected amyloidosis. Since some of the patients had biopsy conducted at two separate sites, the total number of analyzed tissue samples was 452. The investigations conducted at our laboratory included histochemical examination for amyloidosis and possible subsequent immunohistochemical (IHC) amyloid typing.
The tissue samples had been obtained from 178 men and 247 women ranging in age from 24 to 84 years (mean age 62.7 years), with 80% of the patients over 55 years old.
All patients had clinical evidence of amyloidosis, 63% had a concomitant rheumatic condition (these were primarily patients from our Institute), 19% had a concomitant heart condition (patients from the Cardinal Wyszynski National Institute of Cardiology in Warsaw), 5% had a chronic inflammatory condition, 5% were being diagnosed for hematological conditions, and 8% had other comorbidities (e.g. kidney disease, suspected localized amyloidosis, or changes in the respiratory system).
The analyzed specimens were predominantly adipose tissue samples from the abdominal wall (50%), oral mucosa (18%) (half of which contained salivary gland tissues), gastric lining (16%), duodenal and/or large intestinal lining (5%), myocardium (6%), or other structures, such as the urinary bladder, gums, larynx, kidneys, lungs, and a mediastinal tumor (5%).
The received specimens were sliced into 5-micrometer-thick paraffin sections.
Some of the sections from each tissue specimen were then stained with the classic ACR method described by Puchtler [5] and others with the PHCR method described in Japanese by Sai et al. in 1986 and in English by Ishii et al. in 2003 [12]. The stock solution was prepared by adding 45 g of NaCl and 50 g of phenol to 1,000 ml of 50% ethyl alcohol and then filtered. The working solution was prepared immediately before use by dissolving 0.1 g of Congo red in 100 ml of the stock solution and adding 5 g of sodium acetate and 3 ml of acetic acid. Once they had been deparaffinized and rinsed, the tissue sections were stained in working solution for 1 hour. Nuclear staining and dehydration were conducted the same way as in the ACR staining technique.
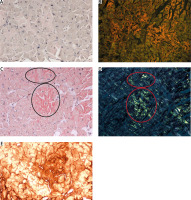
Positive controls were postmortem myocardial tissue sections from a patient who had died of ATTR cardiomyopathy, associated with Ala81Val transthyretin mutation. Negative controls were paraffin sections of skeletal muscle of normal tissue architecture, with no evidence of inflammation. The same tissue sections stained with either method were assessed by two independent pathologists (M. Legatowicz-Koprowska and E. Walczak) in a darkened room, under strong white light and polarized light, using Olympus BX51 microscopes (a 100 W halogen lightbulb) and BX46 (a LED lightbulb) with Olympus polarizers and analyzers. For our semiquantitative assessment we adopted the system of notation from 0 to 3 to indicate deposit quantity, with 0 representing the lack of deposits, and 1, 2, and 3 representing increasing quantities of deposits stained red/pink (with the ACR method) and showing a green glow (apple-green birefringence) under polarized light in crossed Nicol alignment (90°) or stained vivid red (with the PHCR method), with at least partly a green glow under polarized light (green birefringence) (Fig. 1). We would like to emphasize that in the case of PHCR staining, it was only simultaneous vivid red color of the deposits and their bright green glow under polarized light that was considered a positive result. Some of the ACR-stained sections were also assessed under a fluorescence microscope in search for the red/orange glow of amyloid deposits (Figs. 2A, B and 3A, B).
Fig. 1
Lip salivary gland. Amyloidosis AA. A) ACR white light – deposits of amyloid stain red; B) ACR apple green birefringence on polarization; C) PHCR white light – deposits of amyloid stain red – greater extent of deposits visualized via PHCR; D) PHCR apple green birefringence on polarization; E) immunohistochemistry anti-A(+) brown. All ×100.
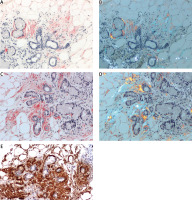
Fig. 2
Gastric mucosa. AL κ in blood vessel walls. A) ACR white light – lack of staining (red ellipses); B) ACR fluorescence microscope – orange fluorescence of amyloid deposits (yellow ellipses); C) PHCR white light – deposits of amyloid stain red; D) PHCR apple green birefringence on polarization (white arrows); E) immunohistochemistry anti-κ(+) brown. All ×200.
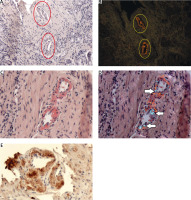
Fig. 3
Myocardium. ATTR. A) ACR white light – lack of staining; B) ACR fluorescence microscope – orange fluorescence of amyloid deposits; C) PHCR white light – deposits of amyloid stainred (black ellipses); D) PHCR apple green birefringence on polarization (red ellipses); E) immunohistochemistry anti-TTR(+) brown. All ×100.
Subsequently, we conducted IHC typing of amyloid deposits into amyloid A (AA), amyloid light-chain (AL), and ATTR, using monoclonal antibodies against serum amyloid A, or polyclonal antibodies against κ -and λ-light chains, and antibodies against transthyretin (all antibodies had been manufactured by DAKO).
The consistency of results of ACR and PHCR methods was expressed in percentage values and Cohen’s κ coefficient values. The interpretation of Cohen’s κ coefficient values is as follows: a value < 0.0 indicates no consistency, 0.01–0.20 indicates none to slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost complete consistency [13].
Results
A total of 452 tissue sections from various organs were analyzed, yielding 283 negative results consistent for both ACR and PHCR methods. Alkaline Congo red staining yielded 94 positive and 358 negative results, whereas PHCR staining yielded 168 positive and 284 negative results. Sections from 75 tissue specimens, which were assessed under white and polarized light, yielded negative ACR staining results but positive PHCR staining results (Figs. 2, 3).
Table I shows a comparison of negative and positive results obtained via either staining method. The percentage agreement for the two staining methods was 83%, with Cohen’s κ coefficient of 0.60 (95% CI: 0.52–0.69), which indicates moderate agreement.
Table I
Comparison of negative and positive results obtained via either staining methods
| PHCR | Negative | Positive | Total |
|---|---|---|---|
| ACR | |||
| Negative | 283 | 75 | 358 |
| Positive | 1 | 93 | 94 |
| Total | 284 | 168 | 452 |
Stratifying the positive results into three groups based on the extent of amyloid deposition (Table II) produced a percentage agreement of 70% between the two methods, and Cohen’s κ coefficient of 0.37 (95% CI: 0.28–0.46), which indicates a fair level of agreement. In 32 of the positive results obtained via both staining methods the extent of deposits received the same rating: 1 in 12 cases, 2 in 5 cases, and 3 in 15 cases. In 61 cases (66%) of positive results obtained via both staining methods the extent of deposits visualized via PHCR staining was rated as greater than that obtained via ACR staining (Fig. 1). Moreover, the intensity of PHCR staining was higher than that of ACR staining, which made the assessment easier (Fig. 1). Some ACR-stained sections were also assessed under a fluorescence microscope, which showed the red/orange fluorescence of amyloid deposits despite a negative result obtained in ACR staining assessed under white light (Fig. 2A, B, Fig. 3A, B).
Table II
The extent of amyloid deposition assessed in the two staining methods
| PHCR | 0 | 1 | 2 | 3 | Total |
|---|---|---|---|---|---|
| ACR | |||||
| 0 | 283 | 37 | 25 | 13 | 358 |
| 1 | 1 | 12 | 16 | 25 | 54 |
| 2 | 0 | 0 | 5 | 20 | 25 |
| 3 | 0 | 0 | 0 | 15 | 15 |
| Total | 284 | 49 | 46 | 73 | 452 |
Histopathology examination findings of sections rated 0 as opposed to those rated 1–3 showed inter-observer agreement (M. Legatowicz-Koprowska and E. Walczak). Sections that received a positive rating showed a very good correlation between the rating pathologists, with an occasional difference (by 1 point) in terms of the extent of deposits.
Seventy-four percent of IHC typing reactions yielded definitive results: 32% AA, 20% AL λ, 9% AL κ, and 13% ATTR (Figs. 1–3). In the remaining 26% of cases, IHC typing yielded inconclusive reactions. IHC typing results were consistent with the clinical presentation, i.e., AA was associated with chronic inflammatory conditions, AL with abnormal serum free light-chain levels or abnormal results of serum or urine protein immunofixation with electrophoresis, and ATTR with the results of genetic analyses or whole-body scans with 99mTc-DPD.
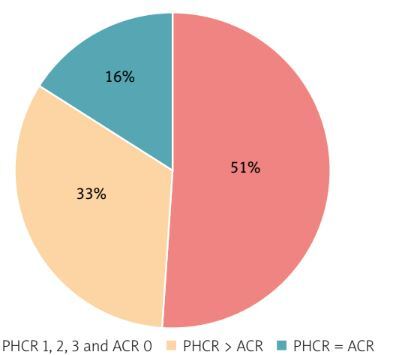
Out of the 51 specimens that yielded ACR 0 and PHCR 1–3 ratings and were further analyzed via IHC amyloid typing, the main amyloid protein was conclusively identified in 42 cases (82.4%).
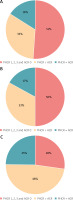
The proportion of consistent and discrepant results obtained with ACR and PHCR staining in the individual amyloidosis types is shown in diagrams (Fig. 4).
Discussion
Conditions characterized by amyloid deposition and the resulting damage to vital organs require early diagnosis to help halt the progression of disease relatively early. Alkaline Congo red staining, also known as Puchtler’s method, still remains the recommended standard (possibly with slight modifications) [5–7] although we should bear in mind that the method is imperfect [14]. Electron microscopy examinations have shown deposits of amyloid-like fibrils in 55% of locations where ACR staining is negative [10]. Pathologists with experience in diagnosing amyloidosis are familiar with the phenomenon of staining variability in cases of AL amyloidosis [10]. It is disturbing that, despite the awareness of the limitations of ACR staining, attempts are rarely made to verify the sensitivity and specificity of various modifications of CR staining in order to increase the detection of amyloid. Personally, having encountered the promising report by Ishii et al. [12] on the high sensitivity of adipose tissue staining with PHCR in patients with AA, we conducted a comparison of the ACR and PHCR staining methods in AA, AL, and ATTR amyloidosis. Cohen’s κ coefficient for agreement between the two methods was 0.60, which corresponds to moderate agreement. Both staining methods yielded consistent results in terms of the lack of amyloid; however, the PHCR staining method showed considerably higher rates of amyloid detection than ACR. In comparison with ACR, PHCR staining demonstrated higher reactivity with amyloid [12]. The use of phenol and sodium chloride is believed to both enhance the amyloid staining intensity and help in its differentiation from the adjacent tissue [12]. The staining protocol includes degradation of connective tissue components at pH 3.0, which makes them lose their affinity for Congo red, while the structure of amyloid remains unaltered [12].
The higher PHCR reactivity with amyloid in comparison with that of ACR observed in our study was present in each of the most common types of amyloidosis, namely AL, ATTR, and AA amyloidosis. Phenol Congo red staining also increased the diagnostic value of biopsy specimens obtained from each site. The specimens in which amyloid deposits yielded negative results in ACR staining would be excluded from subsequent histology examination. This means that, in a real-life setting, these specimens would not be subjected to amyloid deposit typing and no treatment would be initiated.
The notably higher detectability of amyloid using PHCR staining in comparison with ACR staining raises the question of staining specificity. It is important to bear in mind that only bright red staining of the amorphous deposits and their at least partly bright green glow under polarized light (green birefringence) may be considered a positive result of PHCR staining. An unpracticed eye may have difficulty in assessing specimens, such as skin samples, in which all collagen fibers of interstitial connective tissue may stain pale red/pink and have a whitish/greenish glow under polarized light. Such staining of the entire stromal tissue indicates a nonspecific reaction and helps avoid a false positive result. This fact supports the established theory that amyloid detection should be reserved for centers with considerable experience in this field [3].
Our definitive results of IHC typing, which were consistent with clinical data in 82.4% of the analyzed specimens and yielded negative results in ACR staining and positive results in PHCR staining, support the positive result of histochemical PHCR staining. The slightly higher rates of AA than AL (32% vs. 29%) are likely due our laboratory analyzing mainly samples from patients with chronic rheumatic conditions.
Phenol Congo red staining findings include not only a higher intensity (bright red) color of amyloid deposits, but also a greater extent of deposits (in 66% of cases shown in the positive results obtained via both staining methods) (Fig. 1). The described semi-quantitative assessment of ACR and PHCR staining performed with the human eye can be used by pathologists in any histopathological clinical laboratory. This semi-quantitative visual assessment is always subjective and therefore requires examination by two pathologists. Reliability more independent of subjectivity in staining assessments would require digital image acquisition and electronic calculation of the area of the surface stained in a specific color marked by a pathologist. However, it should be taken into account that the extent of amyloid deposits may vary in subsequent tissue sections. A greater extent of deposits may be of importance for the next stage of diagnostics, which involves amyloid typing via mass spectrometry. The first step in this method involves laser dissection of a tissue fragment showing positive histochemical staining (up to now, mainly with ACR) [14]. The use of larger PHCR-positive tissue specimens for mass spectrometry, particularly at the early stages of disease when deposits are minimal, would be of value for the typing process.
Not least importantly, PHCR staining is a simple and inexpensive method, which may be used in screening in patients with suspected amyloidosis as it considerably improves deposit detection.
One limitation of our study is the fact that neither electron microscopy examination nor mass spectrometry typing was used as an additional investigation. Unfortunately, these diagnostic modalities are not available in Poland for amyloidosis diagnostic workup. Conducting future studies involving PHCR staining and subsequent use of either diagnostic modality, or both, to substantiate staining specificity would be of great value.