Introduction
Systemic lupus erythematosus (SLE) is an inflammatory, autoimmune disease characterized by polyclonal activation of lymphocytes, autoantibody production and accumulation of immune complexes which lead to damage of tissue and organs associated with some morbidity and mortality [1]. Due to multiple risk factors (e.g. genetic, hormonal, infection, smoking) SLE has different clinical manifestations and associated production of autoantibodies. Nevertheless, the exact mechanisms which lead to SLE, and actual factors determining which organs are affected, remain less understood.
Interleukin 34 (IL-34) is the second colony-stimulating factor 1 receptor (CSF-1R) ligand and shares the receptor Fms with macrophage colony-stimulating factor (M-CSF) [2]. Remarkably, IL-34 has essential roles in mononuclear phagocyte lineage cell proliferation and diversity, osteoclast production and inflammation [3]. IL-34 has an important role in the pathogenesis of many chronic inflammatory conditions, for example rheumatoid arthritis (RA) [4, 5].
Type I interferons (particularly IFN-α) have a pivotal role in the mechanism of SLE development [6]. A significant percentage of SLE patients presented high serum levels of IFN-α [7]. There are thirteen identified subtypes of IFN-α, so it is challenging to detect IFN-α subtypes in clinical practice. Therefore, some researchers depend on indirect measurements of the IFN signature [8].
Type III interferon group consists of 4 IFN-λ (lambda) molecules: IFN-λ1, IFN-λ2, IFN-λ3 and IFN-λ4 [9]. IFN-λ is an antiviral factor classically formed by virus-infected epithelial cells or plasmacytoid dendritic cells. IFN-λ receptor is expressed mostly on cells of epithelial sources, e.g. skin, gut, kidney epithelium, and neutrophils [10].
Remarkably, the gene signatures of type III (IFN-λ) and type I IFNs overlay [11]. Increased levels of IFN-λ have been found in SLE [12]. Thus, both these IFN subclasses are of interest in the SLE and association with the IFN signature.
There is a theory that diverse pathogenetic pathways and molecules may be associated with SLE subgroups and lead to the observed clinical and serological diversity [13]. Some data have indicated that only a subgroup of SLE patients with the confirmed signature of IFN achieved a good response to treatment [13].
Material and methods
The study included: 82 newly diagnosed SLE Egyptian patients recruited from the Outpatient Clinic of Rheumatology, Rehabilitation and Physical Medicine, Mansoura University Hospital and 60 controls with matched age and gender from our Delta locality (10 males and 50 females with mean age ±SD; 41.6 ±3.2 years).
Medical history, physical examination and laboratory tests for confirming SLE diagnosis and assessment of disease activity such us erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), antinuclear antibodies (ANA) using indirect immunofluorescence on Hep2 cells, double stranded DNA (dsDNA) antibodies, C3 and C4 component of complement were evaluated. Assessment of disease activity was performed using scoring in Systemic Lupus Activity Measure (SLAM) and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Measurement of IL-34, IFN-α and IFN-λ1 serum levels was done using standard ELISA kits (R&D System, Inc. 614 McKinley Place NE Minneapolis, MN55413, USA). All diagnosed SLE patients started immunosuppressive treatment (antimalarial ± steroid ± immunosuppressive drugs) with response evaluation after six months of treatment.
Informed consent was obtained from all individual participants included in the study.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The statistical analysis was performed using the program Excel 2007 and SPSS version 16 (SPSS Inc.). Qualitative data were described in the form of numbers and percentages. Quantitative data were described in the form of mean (±) standard deviation (SD). Statistical analysis was done by comparison between groups using the chi-squared test regarding qualitative data, while quantitative nonparametric data comparison was performed using one-way ANOVA and the paired sample t-test. Survival analysis was calculated by the Kaplan-Meier product-limit estimator. Comparison of survival was performed by the log-rank test; continuous variables were dichotomized at the median cutoff.
The probability of the result occurring by chance (p-value) was calculated for all parameters (p was considered significant at p ≤ 0.05; confidence interval 95%).
Results
Characteristics of study subjects
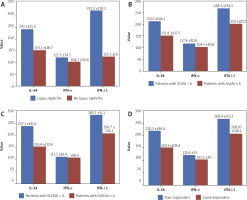
The mean ±SD levels of IL-34, IFN-α and IFN-λ1 in studied patients were 175.9 ±125.9 pg/ml, 109.3 ±32.5 pg/ml and 227.9 ±144.8 pg/ml, respectively. The mean ±SD levels of IL-34, IFN-α and IFN-λ1 in the controls were 24.9 ±1.4 pg/ml, 5.8±2.1 pg/ml and 30 ±4.1 pg/ml, respectively. These levels were significantly higher in the SLE group than in the healthy control group (p ≤ 0.05).
Twenty one SLE patients (25.6%) had confirmed lupus nephritis (LN), ANA positivity was observed in 84.1% (n = 69) patients, anti-dsDNA mean level was 102.4 ±116.8 IU/ml. Other evaluated laboratory tests were as follows: ESR, CRP, C3 and C4 levels were (mean ±SD) 46.7 ±17.7 mm/h, 33.4 ±27.7 mg/l, 104.9 ±60.2 mg/dl and 25.1 ±18.3 mg/dl respectively. There were 39% (n = 32) with SLAM > 6 and 26.8% (n = 22) with SLEDAI > 6. The baseline results in the SLE group and results of studied parameters in the control group are presented in Tables I and II.
Table I
SLE group characteristics and baseline results
Table II
Comparison between baseline levels of serum IL-34, IFN-α and IFN -λ1 in studied groups
The patients started SLE treatment with antimalarial ± glucocorticosteroids ± immunosuppressive drugs, then all laboratory tests were performed after six months of treatment. The test results of follow-up revealed that 55 patients (67.1%) showed good response to treatment while 27 patients (32.9%) were considered as poor responders.
Interleukin 34
Interleukin 34 serum level was positively correlated with the titer of anti-dsDNA (r = 0.33; p ≤ 0.05) but inversely correlated with serum levels of C3 (r = –0.28; p ≤ 0.05) while there were no correlations between IL-34 and age, ESR, CRP, ANA titer and serum levels of C4. A significantly high level of IL-34 was found in patients with LN, with SLAM > 6, SLEDAI > 6 and patients with poor response to treatment. These results are presented in Tables III and IV and Figures 1 and 2.
Table III
Correlations between serum levels of IL-34, IFN-α and IFN -λ1 and other laboratory parameters in SLE group
Table IV
Correlations between serum levels of IL-34, IFN-α and IFN-λ1 and lupus nephritis (LN), SLE activity and response to therapy
Interferon α
Interferon α serum level was negatively correlated with serum levels of C3 (r = –0.43; p ≤ 0.05), but there was no correlation of IFN-α and age, ESR, CRP, ANA titer, anti-dsDNA and serum levels of C4. IFN-α level was significantly high in patients with LN and patients with poor response to treatment, but there was no significant correlation of these cytokine with SLAM > 6 nor SLEDAI > 6 (Tables III and IV, Figs. 1 and 2).
Interferon λ1
Interferon λ1 serum level was positively correlated with anti-dsDNA (r = 0.25; p ≤ 0.05), inversely correlated with serum level of C3 (r = –0.26; p ≤ 0.05), while there were no correlations of IFN-λ1 and age, ESR, CRP, ANA titer and serum levels of C4. IFN-λ1 significantly correlated with LN, SLAM > 6, SLEDAI > 6. A correlation with level of IFN-λ1 and patients’ response to therapy was not observed (Table III, Figs. 1 and 2).
Serum levels of IL-34, IFN-α and IFN-λ1 and accumulation of SLE clinical features
Systemic lupus erythematosus patients were classified into three grades according to the number of clinical features accumulated during follow-up. The correlations between SLE related clinical features and IL-34, IFN-α and IFN-λ1 levels were sought. We found that patients with ≥ 3 accumulated clinical features had a significantly high level of IL-34 (median and 95% CI; 312 [296–367], p ≤ 0.05), high IFN-α (median and 95% CI; 141.5 [123–155], p ≤ 0.05) and high IFN-λ1 (median and 95% CI; 372 [289–456], p ≤ 0.05).
The authors defined patients with high levels (i.e. ≥ 75% or third quartile) of each investigated cytokine, and further grouped the patients into patients with triple high positivity (IL-34 high, IFN-α high and IFN-λ high) versus the rest of patients. We found that cytokine level in patients with triple high positivity (17 patients; 20.7%) was positively correlated with the concentration of antidsDNA (p ≤ 0.05) but negatively correlated with serum levels of C3 (p ≤ 0.05) while there were no correlations with age, ESR, CRP, ANA titer or serum levels of C4.
Triple cytokine level elevation was significantly highly presented in patients with LN, patients with SLAM > 6, patients with SLEDAI > 6 and patients with poor response to treatment (p = 0.01), indicating that these subgroups of patients have a more aggressive disease. There were 28 patients who developed accumulated clinical features (3–8) during the disease course, of whom 15 patients (53.5%) had a high level of the triple cytokines (IL-34 high, IFN-α high and IFN-λ high) also, indicating a poor prognosis of this patient subgroup. Data are presented in Table V.
Table V
Comparison of high level of IL-34, IFN-α and IFN-λ1 (triple positivity) patients and others SLE patients in terms of lupus nephritis (LN), disease activity and response to therapy
Discussion
Interleukin 34 has been described as an alternative colony-stimulating factor 1 receptor (CSF-1R) ligand that is structurally related to CSF-1 but does not have sequence homology with CSF-1 [14]. The site of binding of IL-34 to CSF-1R is on the cleft between D2 and D3 [15]. In addition, IL-34 could bind especially to the extracellular domain of receptor-type protein-tyrosine phosphatase [16] and chondroitin sulphate [17].
Interleukin 34 facilitates the differentiation and survival of monocytes and macrophages, which are the predominant infiltrate in the inflamed synovium and produce inflammatory cytokines such as tumor necrosis factor (TNF) and IL-6 [18]. Additionally, IL-34 itself is able to induce proinflammatory cytokines and chemokines such as IL-6 and IL-8 [19].
Moreover, accumulating evidence suggested that the CSF-1R pathway had a pivotal role in chronic immune diseases such as RA, Sjögren’s syndrome (SS) [20] and SLE with lupus nephritis [21].
Type I interferons (IFNs; particularly IFN-α) play a major role in SLE pathogenesis and a percentage of patients show increased serum levels of IFN-α or IFN-regulated genes’ (IFN signature) upregulation [6, 22]. Patients with SLE usually show increased expression of IFN-α-induced genes in peripheral blood mononuclear cells [23].
This enhanced response is central to the lupus pathogenesis via the differentiation of monocytes into dendritic cells with high antigen-presenting properties [24]. It also regulates B-cell functioning by facilitating the isotype switching to high-affinity immunoglobulin G antibodies [25].
The induction of IFN-stimulated genes by IFN-α may also modulate the expression and signaling of pattern-recognition receptors, chemokines, and other molecules by leukocytes and endothelial cells [26]. Accordingly, a number of drugs targeting type I IFN-related pathways have recently emerged, including monoclonal antibodies that neutralize IFN-α or its receptor, anti-IFN-α antibody-inducing vaccines, and inhibitors of Toll-like receptors [27].
Phase I trials using anti-IFN-α antibodies demonstrated effective inhibition of the IFN-α signature, and preliminary analyses suggested that inhibition of IFN-α could be associated with clinical benefits in SLE [28]. Unfortunately, this initial excitement has been overshadowed by convincing evidence showing no significant difference in the extent of disease activity between anti-IFN-α monoclonal antibodies and placebo, despite a noticeable improvement in a number of laboratory parameters [29]. Therefore, additional contributing mechanisms may also need interference to produce clinically significant changes.
Interferon λ1 is one of four molecules of the IFN type III (IFN-λ) and, interestingly, the gene signatures of type III (IFN-λ) and type I IFNs overlap [11]. Increased levels of IFN-λ were found in SLE [12]. Thus, these IFN subsets are of interest in the context of SLE and the IFN signature. Usually, IFN-λ is developed by epithelial cells infected by virus or plasmacytoid dendritic cells, and there are more sources for IFN-λ such as other antigen-presenting cells, T-helper type 17 (Th17) cells, keratinocytes, and neutrophils [30]. There is a single IFN-λ receptor, which is expressed mainly on epithelial cells, such as skin, gut, kidney epithelium, and neutrophils [9].
In this article, we present our findings on the levels and clinical associations of IL-34, IFN-λ1 and IFN-α in a cohort of SLE patients.
Serum IL-34 level was significantly elevated in SLE patients in previous studies, especially in active SLE patients [31]. In particular, SLEDAI and SLAM were most frequently used to assess SLE disease activity [32].
The present study gives an insight into the relationship between serum IL-34 levels and SLE disease activity, as well as clinical features and treatment response. Serum level of IL-34 positively correlated with SLEDAI, SLAM anti-dsDNA Ab, and C3. Therefore, the IL-34-SLEDAI and IL-34 SLAM correlations suggested that IL-34 is involved in disease evolution. Likewise, anti-dsDNA antibodies are essential to SLE pathology. Thus, IL-34- anti-dsDNA Ab indicated that IL-34 may play an important role in the pathogenesis of SLE.
In this issue our study was in agreement with Wang et al. [31], who found a significant correlation between IL-34 and serum CRP level which did not appear in the present results, and that could be due to the difference in geographic distribution of studied patients.
High levels of IFN-λ1 and IFN-α in SLE patients have been observed in many studies; moreover, they are associated with different clinical and serological profiles of SLE patients. Previously, Wu et al. [12] found that IFN-λ1 mRNA and serum protein were elevated in patients with SLE, especially in subjects with concurrent arthritis or renal disease. In addition, Lin et al. [33] found elevated levels of IFN-λ2 in serum and in transcripts from activated CD4+ T cells of SLE patients compared with healthy individuals. Also high levels of IFN-λ3 were found in sera from patients with serositis and cutaneous disease [34].
In another study, high levels of IFN-λ1, IL-17A, and IL-23 characterized increased organ damage, particularly kidney impairment in SLE patients [35]. A group of researchers in an earlier, smaller Asian study reported that serum levels of IFN-λ1 correlate with SLEDAI and these patients presented renal and/or arthritic manifestations [12].
All these results were similar to our study in that we found that patients with a high IFN-λ1 level were associated with high anti-dsDNA antibodies, LN, and higher disease activity (SLAM > 6 and SLEDAI > 6), confirming the aggressive disease course. We found a high INF-α level in patients with LN although no association with anti-dsDNA, SLAM or SLEDAI was confirmed. This may be attributed to the differences in methods of measurement and the time of SLE activity assessment as we assess the activity before treatment whereas some studies recruited patients already on treatment.
Zickert et al. [36] reported that higher IFN-λ levels in patients with active LN than in control subjects and persistently increased IFN-λ levels were associated to an unfavorable response to treatment. The current study showed that patients with a high pretreatment level of IL-34 and IFN-α demonstrate poor response to treatment while IFN-λ level was not correlated with response to therapy.
As regards these differences, we hypothesized that there may be a new group of patients with high triple cytokine (IL-34 high, IFN-α high and IFN-λ high) levels and this phenotype may herald more aggressive disease. To our knowledge this is the first time in the literature such correlations are described. 17 patients (20.7%) with high triple cytokines also presented higher anti-dsDNA antibody levels, LN and high disease activity measured by SLEDAI and SLAM and also these patients demonstrated poor response to treatment.
These results indicate that this subgroup of patients has a more aggressive disease. Interestingly, 28 patients developed up 3 to 8 accumulated clinical features during the disease course, out of whom 15 patients (53.5%) had a high level all studied cytokines (IL-34 high, IFN-α high and IFN-λ high) also, indicating a poor prognosis of this patient subgroup.
Because of the short follow-up period (6 months), this study could not analyze the relationships between IL-34, IFN-α or IFN-λ and the long-term complications and survival parameters of SLE.
Importantly, clinical trials are evaluating the therapeutic blockage of the IFN-α pathway by the IFN-α receptor (IFN-AR) antagonist anifrolumab as a novel therapy for SLE [13]. The investigators observed that probably not all SLE patients are likely to be candidates for targeting the type I IFN pathway. The present study may provide the explanation that only a subgroup of SLE patients will benefit from IFN-α targeting therapy. Our results may suggests that patients with confirmed high cytokine levels with more aggressive disease could be better candidates for more than one target or a new multi-cytokine target drug in the future.
Conclusions
The present study demonstrated that high pretreatment serum level of IL-34 or IFN-λ1 has prognostic significance in SLE.
Patients with a high serum level of IL-34, IFN-α or IFN-λ1 had more often nephritis and weaker/poor response to treatment.
Especially triple high levels of studied cytokine serum level elevation were significantly associated with LN and high disease activity.
The present results may indicate the need to distinguish this group of patients with such an aggressive phenotype and to consider targeted multi-therapy.