Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune, multisystemic inflammatory disease of unknown origin and characterized by synovial cell proliferation and destruction of the joints by inflammation.
Conventionally, RA is defined as a chronic, inflammatory autoimmune disorder resulting from attacks by the immune system mainly to the articular tissues. It is an impeding and painful inflammatory condition and can lead to significant loss of mobility of the joint due to pain and cartilage erosion. The disease is often systemic because it affects extra-articular soft tissues such as skin, blood vessels, heart, lungs, and muscles of the body. Although it is considered to occur as a result of a multifactorial mechanism, the exact cause has not been elucidated yet.
The administration of bee venom (BV) to experimentally adjuvant-induced arthritis in rats was reported to inhibit pain and destruction of articular cartilage by means of suppressing synthesis of tumor necrosis factor a (TNF-α) and interleukin 6 (IL-6) [1].
At the initial phase of inflammatory arthritis (IA), production of proteinases, inflammatory cytokines and other inflammatory mediators by chondrocytes increases. The cell proliferation rate also accelerates. The relationship between extreme production of inflammatory mediators such as nitric oxide, TNF-α, interleukin 1β (IL-1β) and increase in the synovial fluid resulting from accelerated catabolic process was demonstrated [1, 2].
In recent years, in addition to the disease-modifying anti-rheumatic drugs (DMARDs), TNF-α blockers were also included in the pharmacologic treatment of RA. These blockers reduce or eliminate the symptoms in the acute period of the RA; moreover, in the long term, they might slow down or terminate development of the disease. Such effects cannot be applied to anti-inflammatory drugs and analgesics. The treatment for RA is conventionally initiated with nonsteroidal anti-inflammatory drugs (NSAIDS) and continued with DMARDs and biological agents [2-4]. Although pharmacological agents such as NSAIDs and corticosteroid preparations are used in the current treatment of IA, they fail to provide satisfactory treatment [5].
All of the above-mentioned widely used pharmacological agents were reported to increase gastrointestinal complaints and osteoporosis, and might increase infection risk by suppressing the immune system functions [6]. It is necessary to develop new treatment modalities for pain control, improving functions and quality of life by improving treatment methods with greater effectiveness and lesser side effects in RA treatment.
Altindag et al. [7] investigated the effects of BV on the serum levels of IL-1β, IL-6, TNF-α, high-sensitivity C-reactive protein (hs-CRP), aryl esterase (ARES), osteocalcin and paraoxonase (PON). They reported low serum levels of antiatherogenic enzymes such as ARES and PON in patients with RA. Paraoxonase is an antioxidant enzyme and has a cytoprotective function by contributing HDL activity and by preventing the oxidation of LDL cholesterol. Paraoxonase and ARES may be negatively affected by the oxidative stress which may play a role in the pathogenesis of RA. Based on the results, they claimed that BV might be used in the treatment of RA with its anti-inflammatory effect [7].
Physiological saline (PS) injections have largely been used in establishing placebo controls in experimental studies to distinguish the differences between BV treated and placebo controls. Because PS injected placebo control animals experience lesser pain and no itching in the injection site, the results provide comparison with experimental animals. Also in the present experimental study, PS injections were used as the placebo controls.
The aim of this study is to determine the effects of three different doses of BV on clinical status of arthritis and some serum parameters in experimentally induced adjuvant arthritis. For this purpose, clinical signs of arthritis were followed and serum total antioxidant level (TAL), total oxidant level (TOL), oxidative stress index (OSI) and inflammation mediators, such as IL-1β, IL-6, TNF-α, hs-CRP were determined.
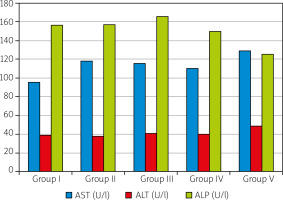
Aspartate (AST), alanine (ALT) aminotransferases and alkaline phosphatase (ALP) were also measured in order to assay possible liver damage.
Material and methods
Animal experiments and laboratory analyses were carried out in Sakarya University Medical Faculty. The research was funded by Sakarya University Scientific Research Projects Coordination Unit (Project No. 2014-08-06-008).
This study was conducted on a total of 35 male adult Wistar albino male rats aged 3 months old weighing 200–250 g. Following a one-week acclimation period, the animals were divided into five groups, each having 7 rats. The animal groups and treatments are given in Table I.
Table I
Animal groups and treatments applied in the experiment
All groups were fed a commercial pellet rat diet and given free access to drinking water for an additional 29 days. The rats were kept at 25°C with a standard 12-hour light and dark cycle. Rats were deprived of food starting from the night of the 29th day. Cardiac blood samples were collected under general anesthesia (ketamine 90 mg/kg + xylamine 10 mg/kg BW) in the next morning.
Experimental adjuvant arthritis was clinically scored in 4 grades: 0 – no swelling, + mild swelling, ++ moderate swelling, +++ severe swelling, ++++ very severe swelling, and the results were evaluated semi-quantitatively [8].
The blood samples were centrifuged at 3000 rpm for 10 minutes for serum collection. Aspartate, alanine aminotransferases and ALP levels were measured by a COBAS-C501 auto analyzer (Roche, Diagnostics GmbH D-68298, Mannheim, Germany). Aryl esterase and PON activities, TAL and TOL levels were measured by means of the methods developed by Erel [9–11]. Oxidative stress index scores were calculated using the TOL/TAL formula [12] and the obtained values were expressed as arbitrary unit (AU).
The serum levels of IL-β, IL-6, TNF-α, hs-CRP were assessed using commercially available enzyme linked immunosorbent assay (ELISA) kits for rats, according to protocols provided by manufacturers (Bender MedSystems, Vienna, Austria). All analyses were made using a micro plate reader (Varioskan Flash, Thermo, USA) and the results were expressed in pg/ml.
Animal care and study protocols applied in the study were in compliance with guidelines of the local Ethics Committee of Istanbul Bezmialem Foundation University (Protocol No. 2015/109, date 31.03.2015).
Statistical analysis
The data were analyzed using IBM SPSS version 22.0 software. The data did not display normal distribution and the significance of differences between mean values of the groups was determined by the Kruskal-Wallis H test. A multiple comparison test, the Dunn-Bonferroni test, was used for determining from which group the significance has arisen. The c2 test was performed to identify the significance of difference among identical groups. A p < 0.05 value was considered as significant.
Results
No clinical signs of arthritis occurred in the group I, whereas severe arthritis symptoms developed in the animals of the remaining groups following injection of Freund’s incomplete adjuvant. In BV administered groups (groups II, III and IV) clinical symptoms of inflammation were relieved (Table II, Fig. 1).
Table II
Semi-quantitative arthritis scores of the groups
| Groups n = 7 | Clinical scores of arthritis and number of animals with each score | |||
|---|---|---|---|---|
| 0 | + | ++ | +++ | |
| I | 7 | – | – | – |
| II | – | – | 4 | 3 |
| III | – | 2 | 4 | 1 |
| IV | – | 5 | 2 | – |
| V | – | – | – | 3 |
| Total | 7 | 7 | 10 | 7 |
Fig. 1
Adjuvant injected sites before (A, C) and after bee venom (BV) treatment (B – 4 μg/kg and D – 20 μg/kg BV injected animals). Significant resolution of the clinical arthritis symptoms was observed in the 20 μg/kg BV-treated group.

Mean serum levels of AST, ALT, ALP, PON, ARES, IL-1β, IL-6, TNF-α, TAL, TOL and OSI of 35 rats included in the study are given in Figures 2, 3 and 4. The levels of inflammatory mediators (hs-CRP, IL-1b, IL-6, TNF-α) were higher in the sham-treatment group (Group V) than in the negative control group (Group I), and the arthritis-induced and BV-treated groups (Groups II, III, IV) displayed declining levels with increasing BV doses. Except hs-CRP level, the levels of inflammatory mediators were significantly (p < 0.001) higher in the sham-treated group (Group V) than the negative control (Group I) and BV-treated groups (Groups II, III and IV). Similar levels of the inflammatory markers were found in both the negative control and BV-treatment groups (Fig. 2).
Fig. 2
Scores of the serum inflammatory mediators of the studied groups.
*The difference between the mean values is statistically significant (p < 0.05).
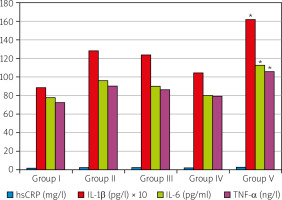
Fig. 3
Serum oxidative stress markers of the studied groups.
*The difference between the mean values is statistically significant (p < 0.05).
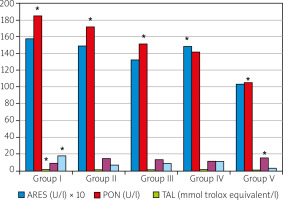
The negative control group (Group I) had the highest level of TAL, which is an indicator of antioxidant level, whereas the arthritis induced sham treatment group (Group V) had the lowest TAL level. The TAL levels of the experimental arthritis induced and BV treatment groups were very similar to each other, and significantly (p < 0.05) higher than that of the arthritis induced and sham treatment group (Fig. 3).
Total oxidant level scores significantly increased in the experimental arthritis induced groups. The negative control group (Group I) had the lowest TOL level, whereas the highest TOL level was found in the arthritis induced and sham-treated group (Group V), and the difference was statistically significant (p < 0.001). In the BV-treated groups, TOL scores were significantly (p < 0.001) lower than the arthritis induced and sham-treatment group, but the 2 µg/kg and 4 µg/kg BW administered groups had similar results, and the level of the 20 µg/kg BW given group had the lowest value among the experimentally arthritis induced groups (Fig. 3).
The levels of PON and ARES were significantly (p < 0.001) lower in the arthritis induced and sham-treated group (Group V) than those of the negative control group (Group I). Although slight decreases occurred in both of the PON and ARES levels of the arthritis induced and BV treatment groups (Groups II, III, and IV) the differences from the negative control group were not statistically significant (p > 0.05) (Fig. 3).
The negative control group (Group I) had the highest (p < 0.001) OSI level among the groups. Experimental arthritis resulted in significant (p < 0.001) declines in OSI scores; however, BV treatment significantly (p < 0.001) recovered the OSI levels. A gradual increase was found in BV-treated groups (Fig. 3).
Liver function enzymes didn’t increased significantly (p > 0.05) and didn’t significantly changed during experimental arthritis induction and the BV treatment. Nevertheless, slight but insignificant (p > 0.05) changes were seen in the ALP activities in the BV-treatment groups (Fig. 4).
Discussion
Rheumatoid arthritis is a typical autoimmune disease, of which exact causative factors have not yet been verified. Although the pathogenesis and pathophysiology of RA have been thoroughly investigated, there are no methods for perfect prevention or permanent cure after onset of RA.
Recently, BV has been proposed as another approach in RA treatment. Several clinical trials have demonstrated that BV therapy could improve arthritis-related symptoms. However, evidence for the therapeutic value of BV is still not sufficient. Although previous researchers [13] suggested that melittin (constitutes 50% of crude honey BV) may be a major causative component of the pharmacological effects of BV, the extent of its improving effects in most clinical and laboratory parameters was similar to or greater than those of BV itself.
Bee venom treatment has been shown to produce anti-inflammatory and anti-nociceptive effects in several animal studies by previous researchers [14–17]. Bee venom treatment might also inhibit proliferation of rheumatoid synovial cells through the induction of apoptosis by caspase-3 activation [17]. In our study, the inflammation-related TAL levels increased, whereas TOS levels decreased. In a previous in vivo experiment [18], researchers showed that anti-nociceptive effects of BV injected into acupoints were greater than those of BV injected into regions other than acupoints.
In a previous study [19], phospholipase A2 was intraperitoneally injected into mice at a dose of 0.2 mg/kg body weight once a day for five days before hepatotoxicity induction with a single dose of acetaminophen (500 mg/kg), a commonly used analgesic and anti-inflammatory drug. Following sacrifice, ALT and AST enzyme profiles, inflammatory cytokines IL-6, TNF and nitrous oxide (NO) scores were assayed. The experimental group animals were treated with a 20 µg/kg dose of BV. Bee venom-treated animals showed markedly decreased hepatotoxic parameters when compared to the non-treated group.
In a previous review [20], the ability of BV, which is a well-established hepato-protective agent, to circumvent hepatocellular toxicity induced by methotrexate was emphasized. BV mitigated the methotrexate-induced hepatotoxicity mostly due to its inhibitory activity on serum TNF-α and tissue NF-κB.
Due to absence of systematical investigations on the safety and effective doses of BV treatment, the severity and frequency of adverse effects have not been comprehensively detailed. In the present study, although the liver function enzyme scores of the BV-treated and sham-treated groups were higher than those of the negative control group, the increases were not statistically significant (p > 0.05). These results might evidence that BV treatment has minimal toxicity towards hepatocytes.
Because subcutaneously administered BV significantly suppresses leukocyte migration and reduces the serum TNF-α concentration, some previous researchers [21] suggested that anti-inflammatory activity of BV is mediated in part by the release of catecholamines from the adrenal medulla. However, Samar et al. [22] claimed that BV exerts its effects by inhibiting proinflammatory metalloenzymes, such as matrix metalloproteinase 2 and 9, and also by increasing interferon β production in a dose and time course dependent manner. Thus, TNF-α plays a pivotal role in the pathogenesis of RA by increasing proliferation of synoviocyte and production of matrix metalloproteinases, causing cartilage degradation [19, 22–24].
In our study, TNF-α was at the highest level in the arthritis induced and sham-treated group. BV treatment decreased TNF-α levels in a dose-dependent manner and the most definite decrease was found in the 20 µg/kg BV-treated group. The decline was at low levels in 2 µg/kg and 4 µg/kg body weight groups. By considering these results, it might be proposed that BV treatment has a modulating effect on the cytokine producing Th1 and Th2 lymphocytes [25].
In a previous study, Mohammadi et al. [26] studied the effects of BV treatment on the cigarette smoke-induced inflammatory (CSC) response in fibroblast-like synoviocytes (FLS). The expression of IL-1β and Sirt-1 was up-regulated even at lower concentrations of BV, whereas it was attenuated at higher concentrations. The researchers concluded that BV treatment attenuated both the CSC and adjuvant induced inflammatory responses in the synovial FLS [26].
It is highly likely that the effectiveness of BV in experimentally induced adjuvant arthritis treatment will be a promising area of future research. Because most of the researchers aiming to determine the role of BV components in the treatment of diseases have used animal models, mainly mice, the benefit of total BV in treating human diseases has not been proven. In addition, studies aiming to determine the optimal effective doses and concentration of BV are recommended for future trials.
In the present study, paw inflammation, which was graded in degrees, recovered in all BV-treated groups when compared to the sham-treatment group. The inflammatory mediator scores of BV-treated groups were also similar to that of the negative control group. However, these clinical and laboratory findings should be confirmed in further trials with larger samples.
Conclusions
The present study provided significant clinical and laboratory evidence on the healing effect of BV treatment in experimentally induced arthritis in the rat model.
The authors hope that the present results are a step before research on a wider study group, which will allow us to assess the possibility of transferring research to humans.