Introduction
Kawasaki disease (KD) is a systemic inflammatory disease and mainly affects children < 5 years old. The etiology of KD is yet to be understood. However, the leading theory is that an unknown infectious agent activates the immune system in a genetically vulnerable child. Kawasaki disease is more prevalent among males and most studies have mentioned a gradual increase in the number of cases from March to June [1, 2].
Geographical location is an important factor in the epidemiology of KD. Prevalence is highest in children of Japanese ancestry and is still increasing (from 218.6 per 100,000 in 2008 to 243.1 and 330.2 in 2011 and 2015, respectively). In contrast, the United States (US) has a relatively stable incidence (the KD-associated hospitalization rate was 18.1 per 100,000 and 19.7 per 100,000 in 2012 and 2003, respectively) [1, 3]. Annual incidence of KD in Gorgan, Iran was 37.39 per 100,000 children [2].
As a systemic disorder with clinical manifestations occurring in almost any organ, diagnosis can be challenging; the guideline from the American Heart Association (AHA) [4] is the most frequently used criteria for the diagnosis and management of KD. Patients who do not fulfill the criteria are categorized as atypical or incomplete [1].
In 2012, the European initiative Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) [5] was established to optimize care for children and young adults with pediatric rheumatic diseases. SHARE has also provided a criterion for the diagnosis and treatment of KD [6]. Although SHARE suggests using AHA criteria for diagnosing typical and atypical KD, other diagnostic and therapeutic aspects are different.
Kawasaki disease is now the leading cause of pediatric-acquired heart disease in developing countries. Complications can occur in different parts of the cardiovascular system, but coronary artery aneurysms (CAA) are the most critical [4].
Although echocardiography is the standard modality of diagnosis for cardiac abnormalities, normal echocardiography never rules out KD [1]. A variety of angiographic techniques such as computed tomographic angiography (CTA) and cardiac magnetic resonance imaging (CMRI) are other available options and are mostly used in patients with coronary artery abnormalities [4]. Other modalities such as ultrasound [7] and lumbar puncture [4] are performed in specific patients.
The main components of the primary treatment of KD are intravenous immunoglobulin (i.v. Ig) and acetylsalicylic acid (ASA). Patients should be treated with i.v. Ig within 10 days after the onset of fever to prevent the development of cardiac complications. Primary prevention of thrombosis is mainly carried out through the application of ASA. Although the application of corticosteroids is well utilized in other forms of vasculitis, its usage in KD is controversial. Surgery in the form of coronary artery bypass grafting and cardiac transplantation is the other therapeutic option, but is usually needed during the late follow-up [1, 4].
Prognosis is primarily based upon the extent and severity of coronary artery involvement at diagnosis and follow-up. The United States has a case-fatality rate of less than 0.2%. The leading cause of death is myocardial infarction resulting from coronary artery occlusion [1].
Half a century has passed since the first report of a series of 50 patients by the Japanese pediatrician Tomisaku Kawasaki [1]. However, controversies still exist in multiple domains of KD, especially about risk factors related to the development of KD. Kawasaki disease is a disease that varies among different parts of the world, and few studies have been performed in Iran. Herein, we conducted a study to determine the characteristics of KD in Kerman, Iran.
Material and methods
Study design and setting
We performed a descriptive, cross-sectional study to determine the basic and demographic data, clinical and paraclinical characteristics and treatments used in hospitalized children with KD between 2007 and 2020.
Study population
In this study the inclusion criterion was having access to the patient’s clinical file. Exclusion criteria were having a simultaneous diagnosis of other rheumatologic disorders, diabetes mellitus, infection or cancer and lacking sufficient data about the patient.
We gathered the clinical files of patients with KD diagnosis. After selecting patients based on the inclusion and exclusion criteria, we obtained the study sample by total population sampling (360 individuals). We extracted the data from their clinical files and entered them into our prepared form. This form consisted of four main components: basic and demographic data, clinical characteristics, paraclinical manifestations and type of treatment. For more concise information, we contacted as many patients as we could and after giving information about the current study and getting their verbal consent, we asked them to give us more detailed answers.
Echocardiographic studies
Echocardiography was performed by a fellowship-trained pediatric cardiologist in two phases: primary investigation and follow-up investigations (2 weeks, 3 months, 6 months). A Z-score < 2 was considered normal.
Results
Basic and demographic data
Our study population consisted of 360 patients (male : female ratio ~ 1.4 : 1). The overall age of our participants was 29.83 ±22.55 months. Most of our patients were between 2 and 5 years old (64.7%). Positive family history (FH) of KD or vasculitis was rare in our population (1 individual with KD, 6 individuals with vasculitis).
The mean ±SD of the duration of hospitalization was 9.89 ±6.11 days, and most of our patients experienced less than ten days of hospitalization (56.4%). A few patients had a history of infectious (10 individuals)/non-infectious (21 individuals) disease. Typical KD was far more common than atypical (316 vs. 44) (Table I).
Table I
Basic and demographic data
Clinical characteristics
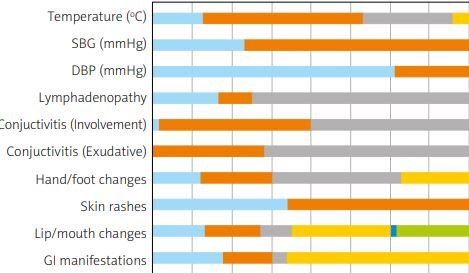
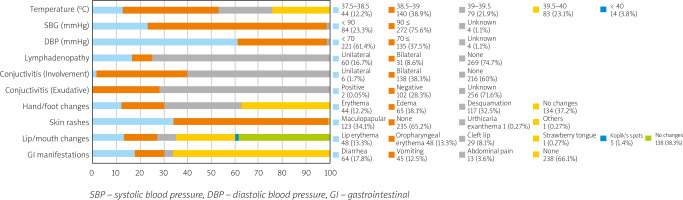
Considering that the forehead is the routine place of temperature measurement, all of our patients were febrile. The overall temperature was also higher than average: 38.03 ±0.87°C. Most of our patients’ temperatures were 38.5–39°C (38.9%). Systolic and diastolic blood pressures (SBP and DBP) were 93.76 ±11 mmHg and 63.66 ±12.25 mmHg, respectively. Most of our patients had SBP of 90 mmHg ≤ and DBP of < 70 mmHg.
No lymphadenopathy was more common (73.4%), but when occurring, the unilateral form was almost twice as frequent as the bilateral form (60 vs. 31 individuals). Conjunctivitis was common, and the bilateral form occurred far more often than the unilateral one (138 vs. 6 individuals). Exudative conjunctivitis occurred in only 2 patients, but it is noteworthy that the presence of exudate was unknown in most cases (71.6%).
The most common finding in the hand/foot was desquamation (32.5%); having no manifestation in the hand/foot was also typical (37.2%). Most of our participants had no skin rashes (65.2%), but when present, the maculopapular pattern was by far the most common finding (34.1%).
The number of patients who experienced lip/mouth involvement was lower than the number of those without it; the most common finding was strawberry tongue, occurring in 25% of the patients; erythema of the lip (13.3%) and oropharynx (13.9%) were other common findings. Having no gastrointestinal (GI) presentation was more common (66.1%), but the most common presentation was diarrhea, occurring in 17.8% of the participants (Fig. 1).
Paraclinical characteristics
Imaging studies
Abnormal chest X-rays were observed in only four patients, and they were in the form of pulmonary infiltration. Ultrasound was performed in 42.1% of the patients, and mesenteric lymphadenopathy (4.2%) was the most common finding.
Echocardiographic findings can be categorized into three domains: vascular (CAA), valvular (insufficiency, stenosis, dilation), and pericardial effusion. Although the most common site of involvement was valvular (33.35%), the most specific finding was CAA (22.5%). In 21.4% of the patients, echocardiography was either normal or not performed. Also, a heart murmur was detected in 4.2% of the population (Table II).
Table II
Imaging studies in the primary investigations
Evaluation of CAAs revealed that the size of the right coronary artery (RCA) and left coronary artery (LCA) was reduced in the follow-up echocardiography compared to the primary one (RCA, 2.01 ±1.78 mm to 1.9 ±0.81 mm, LCA, 2.33 ±0.95 to 2 ±0.67 mm). A total of 175 patients returned for their 6 months follow-up echocardiography and 141 patients (81%) had normal echocardiography; others (34 patients, 19%) had pericardial effusion/valvular insufficiency.
According to the Z-score, primary echocardiography revealed that 18 patients (5%) and 36 patients (10%) had abnormal RCA and LCA sizes, respectively. Follow-up echocardiography showed no abnormal size in any of them.
Laboratory findings
Erythrocyte sedimentation rate (ESR) (95%) and hemoglobin (Hgb) (83.3%) were the two laboratory tests with the highest rates of abnormality. The rates of abnormal C-reactive protein (CRP) (58.9%), aspartate aminotransferase (AST) (64.2%), and alanine aminotransferase (ALT) (50.3%) were also higher than the normal ones, but the difference was slight.
The vast majority of our participants had normal platelets (PLT) (95%), alkaline phosphatase (ALKP) (80.8%), and albumin (ALB) (87.5%). The proportion of patients with normal white blood cells (WBC) was higher than that of patients with abnormal levels by 20% (Table III).
Table III
Laboratory tests
Type of treatments used
All of the individuals in our study received i.v. Ig with a dosage of 2 g/kg and ASA with a dosage of 5 mg/kg. The first dose of i.v. Ig was not effective in 15 patients (4.2%) and the second dose was administered 36 hours afterward. Administration of ASA was continued until the normalization of follow-up echocardiography. Only one patient (0.3%) received steroids in the form of methylprednisolone with a dosage of 30 mg/kg.
A total of 33.7% of our patients received antibiotics: the most common was ceftriaxone in a quarter of our patients, and the least common was amikacin in 1.7% (Table IV).
Discussion
Basic and demographic data
In our study most of our patients were < 5 year old boys and were discharged in under 10 days. This is consistent with previous reports [1, 8, 9].
Only one patient reported FH of KD. Studies [10, 11] have noted opposite results, meaning that paying attention to the FH of KD, especially in the parents, can facilitate the diagnosis.
Our results show that only a minority of patients had FH of vasculitis (1.7%) or a history of non-infectious disease (2.8%). We could not find much data regarding these possible associations, and they need to be addressed in further investigations.
A history of infectious disease was present in 5.8% of our patients. Kawasaki disease and infectious agents, especially respiratory ones, seem to be associated. Studies have mentioned associations between KD and different infectious agents including rhinovirus and respiratory syncytial virus and varicella in the past [12, 13].
The number of patients with typical KD was almost eight times that of patients with atypical KD. The prevalence of atypical KD is unknown, but studies have shown that the prevalence is lower than typical. Fukushige et al. [14] found that 10% of 242 patients in their study had atypical KD.
Clinical characteristics
Although the revised version of the AHA guideline for KD is more flexible regarding the features of fever, it is still the precondition in diagnosing KD. In our population, all of the patients had a fever.
Blood pressure does not usually change in KD. Hypotension can occur in Kawasaki disease shock syndrome (KDSS), characterized by hypotension and shock requiring administering volume expanders and vasoactive agents or transferring to intensive care units [4]. The incidence is estimated to ~ 7% [4]. Studies suggest that KD can be considered a potential source of secondary hypertension [15, 16].
In our study, 25.3% of the patients had lymphadenopathy and the prevalence of unilateral lymphadenopathy was almost two times higher than the bilateral form. In the literature, most of the studies have mentioned lymphadenopathies that are unilateral and confined to the anterior cervical triangle [17].
A total of 40% of our patients experienced conjunctivitis. Rates mentioned in the literature are higher (Cheraghali et al. [2] 51%; Mahmoudzadeh et al. [18] 83%). We believe providing education to patients regarding the prevention of conjunctivitis was a beneficial factor in our study. The number of individuals with bilateral involvement was much higher than the number of patients with unilateral ones (138 vs. 6 cases).
The presence of exudate makes the diagnosis of KD less likely [4]. Our study had the same results: only two cases with positive exudate. Hand/foot changes were present in almost two-thirds of the patients, and desquamation was the most common (32.5%). Mahmoudzadeh et al. [18] reported that 62% of their patients had foot/hand changes.
Skin rashes were experienced by 34.8% of our participants. Except for two cases, all of these skin rashes were maculopapular. A previous report from Cheraghali et al. [2] found skin rashes in 68.6% of the patients. Our results are in contrast with the other studies; the fact that skin rashes are transient manifestations and we only used the initial presentation of our patients could be the potential reasons for this low frequency of skin rashes in our population. Differences in studied populations and the severity of KD are also influential.
More than 60% of the patients presented with lip/mouth changes; strawberry tongue was the most common form of involvement consisting of a quarter of all the manifestations. Cheraghali et al. [2] reported 60.8% of lip/mouth changes in a study on 58 patients with KD. Gradoux et al. [19] conducted a national prospective cohort study in Switzerland; lip/mouth changes occurred in 83% of their 172 patients. Diagnosis of KD is clinical, and lip/mouth changes, especially strawberry tongue, are helpful in this domain.
Gastrointestinal (GI) manifestations are not included in the diagnostic criteria of KD, but studies have reported their presence in KD. Nasiri et al. [7] performed a retrospective, cross-sectional study; they found that vomiting was the most common GI manifestation (28.9%); following that, abdominal pain (17.4%) and diarrhea (16.9%) had the highest prevalence; other manifestations were anorexia, icterus, hepatomegaly, cholecystitis, and gallbladder hydrops. In our study, around one-third of patients experienced GI manifestations, and diarrhea was the most common (17.8%). Although GI manifestations are infrequent, considering KD in a febrile child with GI manifestations is necessary.
Paraclinical manifestations
Imaging studies
Only four patients had chest X-ray involvement; in all of them, the involvement was pulmonary infiltration. Reticulogranular pattern, peribronchial cuffing, pleural effusion, atelectasis, and air trapping are other mentioned involvements in the literature, and most of them are associated with lower respiratory tract inflammation and/or pulmonary arteritis [20].
Ultrasound was performed on 148 patients (most common: mesenteric lymphadenopathy (15 patients)). Hepatomegaly, splenomegaly, pericholecystic halo, and enlargement of the gallbladder are other findings and they mainly correlate with hydrops of the gallbladder [21].
Although SHARE suggests using both echocardiography and electrocardiography (ECG) for all suspected KD patients, echocardiography is the main imaging modality of cardiac evaluation in both AHA and SHARE [4, 6]. In our study, 78.6% of the participants had abnormalities in their echocardiograms. Most studies have shown that 50–70% of patients will have cardiac involvement in the acute phase of KD [22]. The discrepancy between results may be rooted in differences in the accuracy of the operator and paraclinical tools and severity of the disease.
The most common echocardiographic abnormality was CAA (22.5%). The American Heart Association and SHARE both agree that in 25% of untreated cases, CAA occurs due to coronary vasculitis. Valvular disorders, including valvular insufficiency, stenosis, and dilation, occurred in ~ 33.3% of patients. A nationwide survey in Japan [23] reported 20.7% of valvular lesions in their study on 984 KD patients. The lowest ratio of cardiac involvement was for pericardial effusion (9.1%), which agrees with 5% reported by Godfred-Cato et al. [24].
Our study is consistent with literature observing more CAAs in the LCA than in the RCA. Tsuda et al. [25] investigated the distribution of KD-induced CAAs in 204 patients; they divided their participants into four groups based on the maximum CAA diameter (large ≥ 8 mm, medium ≥ 6 and < 8 mm, small ≥ 4 and < 6 mm, very small < 4 mm); they reported that the RCA had more aneurysms than the LCA only in the group with large CAAs. In our study, all of the RCA/LCA aneurysms regressed and there was no sign of RCA/LCA aneurysms in the follow-up echocardiography. The size of the aneurysm is an important factor for the prediction of CAA regression [26]. In our study, the aneurysms were all in the small range (~ 2 mm), which reflects the high chance of CAA regression.
Laboratory findings
Usually, WBC can be normal or elevated [4]; this fact is reflected in our results because the rates of normal (60%) and abnormal (40%) WBCs were close to each other, and the mean ± SD was higher than normal (14,026.3 ±10,812.9).
Hemoglobin had a high ratio of abnormality (83.3%), and the mean ±SD was lower than the normal value (10.38 ±1.54 g/dl). A retrospective study on KD in Spain [27] reported that hemoglobin was lower than normal; this decrease was more significant in atypical KD. Inflammation-induced hepcidin can be impactful in this regard [28].
Thrombocytosis is expected during the second week of KD [4]. In our results, although the mean ±SD was in the upper limit of normal (457,562.7 ±215,518.6), most of the patients had normal PLT (95%). In fact, most of our patients were discharged in under ten days (56.4%), and we assessed the initial laboratory results, which could be the potential cause.
Acute phase reactants will be elevated during the acute phase [4]. The erythrocyte sedimentation rate had the highest percentage of abnormal results (95%), and the mean ±SD was also higher than the normal value (70.45 ±29.3). C-reactive protein was also elevated, but the difference was not as large as in ESR (58.9% abnormal results).
Although the differences were slight, the majority of our patients had abnormal ASTs (64.2%) and ALTs (50.3%). Decreased ALB was present in 12.5% of our patients. Elevated liver enzymes and low levels of albumin are expected in KD [4]. It seems that liver function test abnormalities are common in KD; AST seems to be the most vulnerable; ALKP changes occur infrequently.
Treatment
Intravenous Ig and ASA are the standard initial management of KD [1]. Timely administration can significantly reduce the incidence of coronary artery lesions [1]. In our study, the first dose of i.v. Ig was not effective in a small number of our patients (15 patients, 4.2%) and they received the second dose 36 h after the first one. SHARE mentions i.v. Ig resistance as a strong risk factor for the development of CAA; it also suggests using Kobayashi criteria [29], which are designed to identify i.v. Ig resistance risk; SHARE mentions that in non-Japanese patients, positive Kobayashi criteria may indicate risk of i.v. Ig resistance, but negative Kobayashi criteria may not reliably exclude i.v. Ig resistance.
SHARE also suggests low sodium, raised bilirubin, raised ALT, low platelet count, high CRP and low albumin as predictors of i.v. Ig resistance and by looking at our participants’ laboratory results we can note that most of our patients did not have these risk factors for developing i.v. Ig resistance. This can explain the low rate of i.v. Ig resistance in our study.
The acute phase of KD causes inflammation and platelet activation, and ASA can be helpful in this regard [30]. Although i.v. Ig and ASA are used to prevent coronary artery lesions, they cannot ensure a reduction of this risk [1].
Effective initial treatment consists of a single infusion of high-dose i.v. Ig at 2 g/kg and ASA. In our study, all patients received i.v. Ig with a dosage of 2 g/kg and ASA, which are essential management elements in KD. Although both AHA [4] and SHARE [6] have common suggestions regarding the use of i.v. Ig and ASA, there are some differences between them (Table V).
Table V
Comparison of suggestions of the American Heart Association and Single Hub and Access point for pediatric Rheumatology in Europe for primary treatment of Kawasaki disease
A total of 121 individuals consumed antibiotics, mainly in the form of ceftriaxone (25%), which indicates the close relationship between KD and infections, especially respiratory ones [1].
Worldwide geo-epidemiological differences
Kawasaki disease is a disease with high geo-epidemiological heterogeneity. Asia and particularly Northeast Asia have a significantly higher incidence of KD and all three countries with the highest incidence rates are located in this region: Japan (1 in 65 children develops KD by age 5 years), South Korea (199.7 per 100,000 < 5 years old in 2014) and Taiwan (82.8 per 100,000 < 5 years old in 2010). Also, studies have shown that people with Asian ethnicities have higher KD incidence rates than other ethnicities in a particular region [30–32]. North America also has high KD incidence (17.5–20.8 per 100,000 children < 5 years in the US) [33].
Studies in European countries have reported incidence rates from 1.6 per 100,000 children < 5 years old in the Czech Republic to 17.6 in Italy. Most studies are performed in Northern and Western European countries and data in Eastern European countries are limited. Differences in time frames and methodologies can explain this heterogeneity. The evaluation shows that the annual incidence is about 10–15 per 100,000 children < 5 years old in Western and Northern European countries and seems to be similar to the incidence in Eastern European countries; also, the incidence appears to be relatively stable over time and space in this region [34]. In Iran, Cheraghali et al. [2] reported that the annual incidence of KD in Gorgan, Iran was 37.39 per 100,000 children.
The incidence of KD fluctuates with seasons in different geographical regions: there are winter peaks in Japan [35], winter-spring peaks in the US [36], summer and winter peaks in Beijing and Shanghai, and spring peaks in Sichuan and Hong Kong. Also, in Hawaii studies could not find any clear seasonality [37]. In Iran, Cheraghali et al. [2] reported that most of the KD cases occurred in spring (31.4%), but Asadi-Pooya et al. [38] reported no clear-cut seasonality.
Study limitations
This is a descriptive, cross-sectional study, and like the other studies in this category, it cannot eliminate the effects of confounding factors. Descriptive studies can only show correlation, not causation; therefore, conclusions based on these results should be made with caution.
As geographical location and seasonality influence the prevalence and presentation of KD, we recommend carrying out studies in different locations and times of the year; we also believe that comparing results of different conditions can be helpful. Because KD manifestations can change over time, doing a cohort study with longer follow-ups is also recommended.
Conclusions
Kawasaki disease is a multifactorial disorder that has increased over the past few years. As the leading cause of pediatric heart disease in developing countries, timely diagnosis is the key to starting treatment and preventing complications.
Therefore, considering KD in every febrile child, especially those with risk factors, is essential. Health policies should be focused on appropriate diagnosis and treatment to prevent the occurrence of sequelae.