Introduction
Ankylosing spondylitis, also known as axial radiographic spondyloarthritis (r-axSpA), is a rheumatic disease with a prevalence of ca. 1.5% in the general population [1, 2]. In the course of the disease, the patient’s physical ability becomes considerably impaired due to chronic inflammation within vertebral and sacroiliac joints. Early r-axSpA symptoms usually appear in the mid-20s; the disease occurs in males more often than females [1]. Axial radiographic spondyloarthritis leads to a decline in the quality of life and lessened social and professional activity. As the disease progresses, not only the patient’s physical health worsens, but so does his or her mental health [3]. Concomitant diseases include osteoporosis and depression, among others [3, 4].
Modern biological treatments have had a considerable impact on increasing the life expectancy of r-axSpA patients. In the 1980s, the average life expectancy of r-axSpA patients was markedly lower than that of the general population [5]. Current medications have not only increased the life expectancy but also dramatically improved the comfort of living; they can be considered beneficial from a socioeconomic standpoint [6].
The efficacy of contemporary r-axSpA treatments makes it possible to rapidly achieve low disease activity and, possibly, remission. For this reason, a special focus should be put on the diagnostics and treatment of concomitant diseases, as they also have a meaningful impact on the quality of life of r-axSpA patients.
One such concomitant disease is osteoporosis (OP). During its course, bone mineral density (BMD) decreases, and in a portion of the patients, bone structure disruptions may occur [7]. Consequently, there is increasing risk of so-called low-energy fractures, i.e., fractures that occur during routine actions. Osteoporotic fractures, particularly of vertebral bodies and the femoral neck, incur a significant loss of quality of life and increased risk of premature death.
In the case of r-axSpA patients, the prevalence of OP and fractures as a result of r-axSpA is significantly higher than in the general population [8]. It is estimated that vertebral fractures alone may affect up to 30% of patients with r-axSpA [9]. The frequency of fractures and the severity of their consequences increase with age [10].
Additionally, due to the course of the underlying disease, OP develops at an earlier age compared to the general population. This makes OP a diagnostic and therapeutic challenge in r-axSpA patients.
The relation between r-axSpA and OP is complex. The course of r-axSpA in and of itself may have an impact on the increased frequency of osteoporotic fractures on a biochemical basis, i.e., elevated synthesis of inflammatory cytokines, diminished physical fitness, and loss of muscle mass – the development of sarcopenia [11–13].
The dual X-ray energy absorptiometry (DXA) test is the gold standard in the diagnostics of OP in the general population. In DXA, fracture risk is assessed based on a BMD measurement. In the case of patients with r-axSpA, fracture risk assessment based solely on BMD is insufficient, however [8]. In the early stages of the disease there is a decline in BMD, but later on BMD rises due to the emergence of syndesmophytes, among other factors [14, 15]. For this reason, in order to assess fracture risk in r-axSpA patients, it is imperative to extend the diagnostics with bone structure assessment [8, 16].
Bone structure may be key to accurately assessing fracture risk, should BMD abnormalities emerge in the course of the disease. Fracture risk assessment in patients with diabetes may corroborate this proposition – in this group, BMD is markedly higher than in the general population, yet osteoporotic fractures occur more often [17]. The increased fracture prevalence may be explained based on bone structure abnormalities compared to the general population [17].
The same situation applies to r-axSpA, further aggravated by syndesmophyte growths. Considering this, BMD assessment within the lumbar spine in DXA testing could be omitted and fracture risk calculated based on the femoral neck alone. In many cases, however, due to the young age of the patients, this test may be unreliable. A decrease in BMD of the femoral neck usually occurs in a later stage of life. A decline in BMD in the femoral neck is usually observed only after the age of 60. For these reasons, it is imperative to extend the diagnostics for OP with r-axSpA patients. Fracture risk assessment based off BMD alone may produce false negatives [8].
The aim of this paper is to draw attention to the issue of OP in the course of r-axSpA and to discuss diagnostic methods for OP.
Consequences of osteoporosis
Body mass densitometry changes in the course of r-axSpA develop in a distinct way. In the early stage of the disease, due to the inflammation, there occurs a decline of BMD in both the femoral neck and spine [13]. Yet as the disease progresses, BMD increases on account of osteogenic processes related to r-axSpA [13].
Reduced physical fitness and sarcopenia in r-axSpA patients raise the risk of falling and therefore osteoporotic fractures. What is more, the increased risk of falling may also lead to symptoms of depression [18], related to the diminished quality of life of the patient. It could be claimed that, indirectly, the consequence of OP in the course of r-axSpA is deterioration of not only physical health, but also mental health [18]. Lastly, fractures, particularly cervical fractures, are strongly associated with elevated mortality risk in r-axSpA patients [19].
As a result, with modern treatments for r-axSpA, management of OP may be the core factor that will improve the patient’s physical and mental condition.
Diagnostic methods
Osteoporosis diagnostics is currently not limited to DXA; there are several other means of fracture risk assessment that can be used with r-axSpA patients.
Computed tomography
Among currently available testing methods for assessing osteoporotic fracture risk, worth mentioning are methods based on computed tomography, i.e., quantitative computed tomography (QCT) and peripheral QCT (pQCT), their distinct feature being very high sensitivity in OP diagnostics compared to DXA [20].
These are not the first-choice methods from a clinical standpoint, however, the primary limitation being their low availability at the present time. Moreover, there are no commonly established guidelines regarding the interpretation of the results and their application in the ongoing treatment.
According to the definition by the World Health Organization (WHO), it is been accepted that OP is diagnosed for T-score values lower than or equal to –2.5 in the DXA [21]. This concerns postmenopausal women and men aged 50 and older if the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or less. In the case of premenopausal women and men under 50 years old, there is no consensus on the diagnosis of OP. According to the 2023 official position of the International Society of Clinical Densitometry (ISCD), the Z-score, not the T-score, should be used [22]. In this case, ISCD defines a Z-score of –2.0 or lower as “below the expected range for age” and a Z-score above –2.0 as “within the expected range for age”. In this patient group, OP cannot be diagnosed solely based on BMD; fragility fractures or secondary causes of OP are necessary for diagnosis [23]. The International Osteoporosis Foundation (IOF) recommends using a Z-score below –2.0 to define low bone mass in children and adolescents (up to age 20) [23]. However, unlike ISCD, in the age group of 20–50 years, the IOF recommends using the T-score and diagnosing OP for a T-score ≤ –2.5, particularly in cases of fragility fractures or secondary causes [23].
It should be noted that DXA calculates the so-called aerial BMD (aBMD), measured in g/cm2, whereas QCT/pQCT tests calculate volumetric BMD (vBMD) measured in g/cm3. As a result, the different measurement methods make it difficult to compare the two results. In addition, QCT/pQCT tests do not consider deformative changes within cortical bone, which allows OP to be diagnosed more easily than with DXA [20]. It is presently accepted that OP is diagnosed in QCT/pQCT tests for vBMD values lower than or equal to 80 mg/cm3.
It should be emphasized that in QCT/pQCT tests, the dose of ionizing radiation to which the patient is exposed does not present a serious issue. Bone density can be assessed during computed tomography performed for other medical reasons. Similarly, during pQCT scans only limbs are exposed, providing only a light strain for the patient [24–27].
Radiofrequency echographic multi-spectrometry
Radiofrequency echographic multi-spectrometry (REMS) is a relatively new method of bone density assessment, performed through the analysis of ultrasound signals against a predefined model [28]. The test utilizes a convex head that emits a wave with a frequency of 3.5 MHz. This allows one to assess bone density in both the femoral neck and lumbar spine. The test is carried out in a fashion similar to a standard ultrasound scan.
The latest research results suggest that REMS might be a useful method for fracture risk assessment with secondary OP, e.g., patients with diabetes [29]. In patients with rheumatoid arthritis (RA), REMS might also be a method superior to the standard DXA scan [30].
Unfortunately, to the best of our knowledge, no research data concerning the use of REMS on patients with r-axSpA have been published. However, considering the usefulness of this method in cases with secondary OP, there is hope that the REMS test will make it possible to reliably measure fracture risk in r-axSpA patients.
Dual energy X-ray absorptiometry
Many researchers incorrectly see the DXA test as a means of assessing risk fracture based exclusively on BMD. In actuality, this was proven false years ago. Modern densitometry is not merely a physical measure of bone density. With the use of numerical methods, advanced software also allows one to assess the bone structure and calculate the risk of osteoporotic fractures. In the case of the lumbar spine, the trabecular bone score (TBS) can be assessed thanks to, for example, the Insight program; in the femoral neck it is possible to differentiate between cortical and trabecular bone with the 3D-Shaper software [7, 31]. It bears repeating that these are strictly numerical methods – similar to BMD assessment in the REMS test – and not an actual physical measurement.
Trabecular bone score
The TBS is a numerical method that allows one to assess the bone structure in a DXA test [7]. An effort to introduce it into routine clinical practice has been ongoing for over a decade. The TBS assessment compares BMD distribution across individual pixels within a given circle. The more even the distribution is, the higher is the TBS score and therefore the lower is the risk of an osteoporotic fracture.
As for the practical application of TBS, the method is presently in a unique position. On one hand, a number of studies have proved its usefulness in identifying both patients with fractures and those with an elevated risk of fractures. On the other, there is a lack of guidelines allowing the use of this method in routine clinical practice [8, 32–34]. The official position of the ISCD from 2023 is an example of this issue. It highlights the diagnostic value of TBS, yet from a clinical standpoint it cannot be considered a leap forward compared to the previous position from 2019. Needless to say, the current official position of the ISCD from 2023 states that the monitoring and reporting of TBS changes in routine clinical practice are not recommended for treating patients with OP.
To introduce TBS into routine clinical practice to monitor the efficacy of the treatment would require many years of monitoring patients, a fact that could have informed the aforementioned ISCD recommendation. So far, in the overwhelming majority of cases, we have dealt with retrospective studies, including the largest-scale one – the Manitoba BMD Registry, which, among other things, assessed fracture risk in patients with type 2 diabetes [34]. It is worth noting that the ability to carry out the study in a retrospective manner allowed the Manitoba BMD Registry to assess ca. 30,000 patients, and thus produce reliable data regarding TBS scores in the context of osteoporotic fractures.
The introduction of TBS to fracture risk assessment may also contribute to increased efficacy of next generation medications. Current medications, including the highly effective denosumab, mainly elevate the BMD score, but the TBS shows that their impact on bone improvement is not as high.
In the case of patients with r-axSpA, TBS assessment may be more important than in the general population. In this group, fracture risk assessment based solely on BMD in DXA tests is insufficient (Figs. 1, 2).
Fig. 1
27-year-old patient 1 with r-axSpA. The DXA examination shows normal results, which may be a consequence of the course of r-axSpA.
BMC – bone mineral content, BMD – bone mineral density

Fig. 2
27-year-old patient 1 with r-axSpA. Trabecular bone score assessment indicates degraded microarchitecture.
TBS – trabecular bone score.

In the course of r-axSpA, the initial loss of BMD as a result of chronic inflammation is subsequently masked by osteogenic processes. Furthermore, at the same time the risk of falling increases and so does the risk of fracture due to the growing stiffness of the sacroiliac joints and spine. This, in turn, leads to reduced physical activity, which, combined with the disease, may reduce muscle tissue, and, in some cases, to sarcopenia. At the biochemical level, there also applies an intensified synthesis of interleukins (IL) deriving from the course of r-axSpA itself, which may also contribute to the decline in BMD [35].
Through the inhibition of IL synthesis, modern biological treatments can bring about an improvement in not only BMD but also bone structure, as proven by a clinical study on patients with RA [36]. Biological medications used in treating r-axSpA can exhibit a similar effect on the osseous system as well. Consequently, in patients with r-axSpA, BMD and TBS can be affected by medications for both the underlying disease and OP, which makes it difficult to determine the efficacy of treatments when dealing with both of these diseases at once. Furthermore, reduced activity of the underlying disease and enhanced physical fitness result in a lowered risk of falling and thus risk of fracture.
For these reasons, determining which factor has the decisive impact on the improvement of TBS and frequency of fractures in r-axSpA patients is a challenge. Consequently, it is difficult to utilize TBS as a basic element to monitor the efficacy of an ongoing treatment. At present, the use of TBS depends primarily on the clinician’s experience, despite the fact that a number of studies have proved the worth of this diagnostic method in r-axSpA patients [8, 37].
Regardless, the challenges outlined above do not undermine the usefulness of this method or the added value of standard BMD assessment in DXA testing. There is significant agreement in the results between research centers. Considering the high precision of measurement and the ubiquity of DXA, it appears that TBS measurement may become the most important in treating OP in r-axSpA patients compared to other methods of measuring bone density and bone structure. However, it seems that collaboration between research centers is imperative in order to assess a larger group of patients in a shorter timeframe, producing reliable conclusions regarding TBS measurement in that group.
Dual X-ray energy absorptiometry – total body measurement
Dual X-ray energy absorptiometry scan may also be used to conduct a total body composition analysis. This test is, naturally, not used for measuring bone density and fracture risk. However, from the perspective of OP in the course of r-axSpA, its usefulness in this context cannot be ruled out. Body composition analysis combined with a simple grip strength test using a dynamometer may identify patients with sarcopenia. Sarcopenia can be considered a significant factor in elevated risk fracture [38]. Due to the reduced physical activity of r-axSpA patients, it is also worthwhile to consider the possibility of developing sarcopenia, whose prevalence may be higher than in the general population. It is estimated that approximately 35% of patients with r-axSpA also develop sarcopenia [39]. Such a high prevalence of sarcopenia is associated not only with reduced physical activity and fear of falling, but also with pro-inflammatory cytokines such as tumor necrosis factor (TNF), IL-6, and C-reactive protein [39–41].
As a result, while a total body test with the DXA method cannot be considered routine in fracture risk assessment, in r-axSpA patients it can serve as an auxiliary method to evaluate the additional risk factor that is sarcopenia. Unfortunately, the proper application of the results of this test depends solely on the clinician’s experience.
Preventing sarcopenia in r-axSpA may be aided by increasing physical activity through rehabilitation and home exercises, as well as a diet rich in protein, among other nutrients [42, 43].
Software for parametric 3D modeling
The previously outlined TBS program is limited to assessing the bone structure within the L1–L4 vertebra, leaving a diagnostic gap of sorts, as it does not allow one to assess the bone structure of the femoral neck, which is also an important test from the perspective of fracture risk assessment.
The 3D-Shaper is relatively new software that fills that gap in DXA testing. Similar to TBS, it requires no alterations to the testing procedure and allows for retrospective analyses.
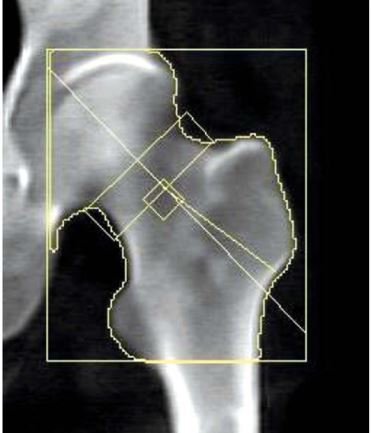
Based on a standard DXA scan of the femoral neck, the algorithm allows one to differentiate between cortical and trabecular bone, making it possible to bypass degenerative changes in the analysis, present primarily in the cortical bone, and subsequently adjust the patient’s medication depending on which bone type suffered a greater decline in BMD (Figs. 3, 4).
Fig. 3
50-year-old patient 2 with r-axSpA. Standard BMD assessment of the femoral neck.
BMC – bone mineral content, BMD – bone mineral density.

Fig. 4
50-year-old patient 2 with r-axSpA. Evaluation considering the separation of cortical and trabecular bone using the 3D-Shaper software.
sBMD – subchondral bone mineral density, vBMD – volumetric bone mineral density.

To the best of our knowledge, there have been no study results published concerning the use of this method of fracture risk assessment in r-axSpA patients. However, there was a study that involved the use of 3D-Shaper in patients with RA [44].
Brance et al. [44] demonstrated that it is possible with 3D-Shaper to track structural changes in the femoral neck stemming from long-term use of both glucocorticosteroids (GC) and biological medications. The observed changes were also related to the duration of the disease. Considering that both GC and biological treatments are used in r-axSpA patients, similar results are expected in this case too.
As it stands, how the data provided by 3D-Shaper are utilized closely depends on the clinician’s experience. As with TBS, there are no official guidelines regarding the use of such an assessment in the diagnostics and monitoring of patients with OP. Regardless, preliminary study results are promising. In addition, considering the wide availability of DXA machines, there is an even greater incentive to verify the usefulness of the software in the diagnostics and monitoring of OP in patients with r-axSpA.
Conclusions
From the standpoint of routine clinical practice, OP constitutes a considerable problem in r-axSpA patients. Due to the course of r-axSpA, both diagnostics and monitoring of the efficacy of the ongoing treatment continue to present a major diagnostic challenge.
At present, the DXA test appears to be the most rational choice due to its wide availability. However, it is imperative to extend fracture risk assessment by parameters related to bone structure. Assessment based on BMD alone is insufficient. Therefore, for a DXA scan to have any diagnostic worth, it is necessary to utilize additional software to measure bone structure in the femoral neck and spine, e.g., TBS and 3D-Shaper, respectively.
The lack of guidelines on bone structure means that even with additional software, the therapeutic procedure will depend on the clinician’s experience, and it is unfortunately very unlikely that this situation will change in the foreseeable future. A multicenter study with a prolonged observation period would be necessary for that.
Due to their limited availability, the other diagnostic methods outlined above have presently little chance of being adopted in routine clinical practice, despite their high sensitivity in OP diagnostics in patients with r-axSpA.
In conclusion, for the purposes of assessing fracture risk in r-axSpA patients, the emphasis should be placed on bone structure evaluation instead of BMD. It is currently possible in DXA, but requires additional software. Educating clinicians on this topic is paramount so that DXA tests are not associated exclusively with BMD, and to draw up consistent recommendations regarding the interpretation of bone structure during treatment.