Introduction
Systemic sclerosis (SSc) is a connective tissue autoimmune disease, characterized by damage of blood vessels and progressive fibrosis of the skin and internal organs. Though the etiology of SSc remains unknown, both genetics and environmental factors may play some role in the pathogenesis of SSc. One of the known environmental factors is silicon dioxide [1]. However, not only crystalline silica may trigger an autoimmune response. Different silicon compounds were also described to stimulate autoimmune reactions in the human body.
A possible causal association between silicone breast implants (SBI) and connective tissue disease (CTD) has been suggested since the 1960s, soon after the introduction of SBI [2]. The first case reports of SSc after augmentation mammoplasty emerged in 1979 [3]. Although the relationship between SSc and SBI was intensely investigated, no clear evidence of such an association was ever found [1, 4, 5]. It is now proposed instead that SBI may cause non-specific autoimmune symptoms without meeting the diagnostic criteria of any specific autoimmune disease. This phenomenon was named siliconosis, a part of the autoimmune syndrome induced by adjuvants (ASIA syndrome) [6].
ASIA syndrome is worth considering in the differential diagnosis in rheumatology patients. In support for this opinion, we present a case of a scleroderma-like syndrome in a 48-year-old woman with a broken silicone breast implant.
Case report
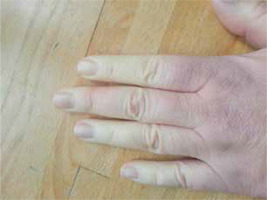
A 48-year-old woman presented with Raynaud’s syndrome (Fig. 1) lasting for 3 years and chronic unproductive cough for 5 years. Additionally, she reported dyspnea and fatigue for over 1 year, and from about 2 months she complained of difficulty with swallowing and choking during meals. In laboratory tests performed on an outpatient basis antinuclear antibodies in a titer of 1 : 320 with anti-Scl-70 antibodies were found. Based on those findings the patient was directed to our rheumatology department for further evaluation.
Investigation of her past medical history revealed augmentation mammoplasty 11 years before her first symptoms. On admission, physical examination did not reveal any abnormalities other than Raynaud’s syndrome and puffy fingers.
In laboratory tests, we detected antinuclear antibodies in a titer of 1 : 640 in a homogeneously speckled and nucleolar staining pattern. The qualitative test panel for systemic sclerosis-associated antibodies showed anti-Scl-70 antibodies in a high titer (+++) and anti-RP155 antibodies in a low titer (+). No additional abnormalities in laboratory tests were found. Despite clinical symptoms of Raynaud’s syndrome, nailfold capillaroscopy showed a normal capillary pattern. X-ray examination with barium contrast demonstrated no abnormalities in the upper gastrointestinal tract. Lung function testing showed no ventilation disorders – vital capacity was 102% of predicted value and diffusing capacity for carbon monoxide 92.5% of predicted value. Echocardiography demonstrated no evidence of pulmonary hypertension nor any other abnormalities. High resolution computed tomography of the thorax revealed no pulmonary fibrosis, but detected inhomogeneous density with spiral structure in the left breast implant, suggesting a broken implant capsule. The patient was aware of this problem, as 3 years earlier in ultrasound breast examination inhomogeneous echogenicity with streaks that may correspond to a broken implant was found, but due to economic reasons, she postponed reimplantation.
Based on all clinical findings we diagnosed scleroderma-like syndrome induced by SBI in the course of ASIA syndrome. We recommended removal of the breast implants, but at that time no pharmacotherapy directed toward autoimmune disease was introduced. As early stages of SSc could not be excluded, the patient was referred for active surveillance in our outpatient clinic.
The patient removed both SBI after a few months. One year later the chronic cough and difficulty with swallowing have resolved, but dyspnea has persisted and Raynaud’s syndrome has intensified. Meanwhile, the patient has been diagnosed with Hashimoto’s thyroiditis. The titer of antinuclear antibodies has risen to 1 : 1280. Also, anti-citrullinated protein antibodies were detected (40 U/ml), as well as proteinuria (0.3 G/l) and persistent microscopic hematuria (up to 16 RBC/HPF). Due to disease progression, hydroxychloroquine therapy was initiated. The patient was also referred to a nephrologist.
Discussion
The mechanism of autoimmunological reaction induced by SBI is still elusive. It has been proposed that in patients with SBI, foreign body reaction stimulates CD68+ macrophages and CD4+ T-cells to form a periprosthetic capsule around the SBI. In persons with a genetic predisposition, IL-6, TGF-β and Th-17 cells may convert an acute reaction into a chronic reaction, which may trigger systemic disease [7]. Some studies suggest that vitamin D deficiency [8, 9] and pre-existing allergies [9] may be risk factors for the development of autoantibodies in patients with SBI. “Bleeding” of silicone outside the SBI capsule may further increase the risk of an autoimmunological reaction. The leakage of silicone from implants was reported in one study to increase the risk of self-reported CTD [10] and in another study increased development of antinuclear antibodies [11].
The postulated causal association between SBI and development of SSc was intensively studied in the 1990s, but at that time was not confirmed. All meta-analyses performed until 2000 showed no evidence of an association of SSc or any other CTD with SBI [4, 12–15]. However, the most recent meta-analysis by Rubio-Rivas et al., performed in 2017, showed increased risk of SSc in patients with SBI when case-control studies were taken into consideration (relative risk [RR]: 1.68, 95% confidence interval [CI]: 1.65–1.71), but no increased risk in cohort studies (RR: 2.13, 95% CI: 0.86–5.27) [1]. This discrepancy may result from the fact that for rare outcomes, such as SSc occurrence, case-control studies may better identify the risk than cohort studies.
Further important evidence regarding the safety of SBI comes from just published largest cohort study to date by Coroneos et al. [5]. Nearly 42,000 patients with SBI were analyzed and SIR of SSc was calculated to be 7.00 (95% CI: 5.12–9.34) [5]. The data come from post-approval studies conducted in the USA on behalf of the Food and Drug Administration. Unfortunately, although original data covered nearly 100,000 people after mammoplasty, including over 83,000 people with SBI, due to low compliance, analysis of SSc occurrence was performed on a small proportion of the population. The data were also based on self-reporting, which may be a significant limitation. The RR of self-reported CTD after mammoplasty augmentation was found to be significantly increased in cohort studies by Brinton et al. (RR: 2.0, 95% CI: 1.5–2.8) [16] and Lee et al. (RR: 1.6, 95% CI: 1.28–2.0) [17], but the risk disappeared when medical records were taken into consideration.
Although the question whether SBI triggers SSc is still without a definitive answer, it was shown in previous studies that patients after augmentation mammoplasty may present different nonspecific autoimmune reactions and symptoms not fulfilling diagnostic criteria for the defined CTD. Several studies have reported an increased incidence of Raynaud’s syndrome [16, 18], the presence of antinuclear antibodies [19, 20] and Scl-70 antibodies [21] after SBI mammoplasty. Other studies have reported significant increases in many unspecific symptoms such as arthralgia, myalgia, chronic fatigue or impaired cognition [9, 22, 23].
In 2011, Shoenfeld and Agmon-Levin proposed that SBI may trigger an autoimmune reaction in genetically predisposed subjects leading to a collection of different unspecified symptoms. They called it siliconosis, and proposed it to be a part of the ASIA syndrome [6]. Colaris et al. [24] in their literature review found 18 cohort studies of SBI patients fulfilling proposed ASIA criteria.
Our patient did not fulfill the diagnostic criteria of SSc, but she met the criteria for recognizing the ASIA syndrome. We suggested removal of our patient’s breast implants, as it was shown in the systematic literature review (23 studies, 622 patients included) that up to 75% of complaints related to SBI disappear after implants’ explantation [25]. However, the study by Colaris et al. [24] showed that in 26% of patients the improvement after SBI removal was only temporary.
It may be partially due to psychological distress of women requesting removal of silicone breast implants [26]. In the literature review, only 16% of patients had improvement of symptoms when they were diagnosed with the defined autoimmune disease and did not receive additional immunosuppressive therapy. Removal of SBI also did not have an effect on autoantibodies [25]. That is what happened to our patient. Only part of the symptoms subsided and autoimmunization increased – ANA titers increased and the patient developed anti-citrullinated protein antibodies and was diagnosed with Hashimoto’s thyroiditis. She remains under further rheumatological observation.
Conclusions
Our case along with other cases and epidemiological studies shows that patients with SBI may present with various unspecific symptoms and immunological abnormalities, which may cause the patient to be referred to the rheumatologist. Physicians should be aware of the fact that these cases may resemble different CTDs.