Introduction
Idiopathic inflammatory myopathies (IIMs) are a rare, systemic, and heterogeneous group of inflammatory diseases characterized by chronic or subacute proximal muscle weakness, tenderness, and myalgia. The Bohan and Peter classification criteria including symmetrical muscle weakness, elevated serum muscle enzymes, electromyographic changes, characteristic muscle biopsy findings, and typical skin eruptions are commonly used for diagnosis [1].
Typical skin rashes such as heliotrope rash, Gottron’s papules/sign, the V-sign, and shawl sign may be seen in dermatomyositis [2]. Levels of the muscle enzymes serum aldolase, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels are increased.
The most sensitive muscle enzyme is serum CPK, which increases in the acute phase, which may increase up to 10–100 times in IIMs, and is used in the clinical follow-up of patients due to its correlation with disease activity. In addition, myoglobulin, found in skeletal and cardiac muscle, may rise earlier than CPK and correlate with disease activity [3].
The Myositis Intention to Treat Index and MYOACT are used for the severity of damage and to evaluate the disease activity of various organs and systems in myositis. Scoring systems include structural symptoms, skin, musculoskeletal, gastrointestinal, pulmonary and cardiac findings [4]. In addition, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may increase in the active phase of the disease [3].
In rheumatologic diseases, inflammation has an effect on hematopoiesis and may lead to sequelae such as anemia and thrombocytosis. The changes are associated with cytokines such as IL-1, IL-3, IL-4, IL-6, and tumor necrosis factor α (TNF-α), which play a role in pathogenesis [5].
As a result of the disruption of erythropoiesis in inflammation, smaller volumes of erythrocytes are added to the circulation and the red cell distribution width (RDW) value is determined to be higher. In addition, as the neutrophil count increases, the lymphocyte count decreases, and the neutrophil/lymphocyte ratio increases [6].
Because inflammation and disease activity are correlated in inflammatory myositis, it seems reasonable to use these parameters as biomarkers in detecting disease activation. The levels of platelet parameters such as MPV, PDW and plateletcrit may change in many inflammatory conditions such as infections, malignancies, cardiovascular diseases, and rheumatic diseases [5].
However, there are limited data on PDW in rheumatic diseases, especially in IIMs. In the present study, we aimed to evaluate the PDW as a disease activity marker and the laboratory and clinical characteristics of IIMs.
Material and methods
Participants
A total of 27 patients with IIM and 30 healthy controls aged 18 years and over were included in the study. The Peter/Bohan classification criteria was used for the diagnosis of IIMs [7].
Patients were excluded from the study if they had infections, malignancies, hematological disorders, metabolic myopathies, certain muscular dystrophies, drug/toxin-induced myotoxicity, neuropathies, and other rheumatic diseases such as rheumatoid arthritis, Sjögren’s syndrome, etc. The study was designed as single-center, analytic, descriptive, and cross-sectional.
Demographic and clinical features
Age, gender, medications, clinical findings, ESR, CRP, LDH, AST, ALT, and CPK were evaluated. Hematological laboratory parameters including platelet count, PCT, mean platelet volume (MPV), and platelet distribution width (PDW) were measured within one hour of blood collection.
The normal reference range for PCT, MPV, and PDW was accepted as 0.18–0.39, 9.4–12.3, and 9.9–15.4, respectively. Myositis Intention to Treat Index and MYOACT were used to evaluate the involvement and severity of involvement of seven target organs for IIM in the last four weeks [8]. Myositis disease activity assessment Visual Analogue Scale scores were recorded for each of the constitutional, cutaneous, musculoskeletal, gastrointestinal, lung, and cardiac involvement.
In the MITAX score, each system is evaluated separately as: score 0 – no involvement, score 1 – improvement, score 2 – stable course, score 3 – worsening, and score 4 – new clinical presentations.
Also, organ involvement is classified into 5 groups from A to E. Group A defines active involvement to use disease-modifying agent, B – moderate activity, C – under control with symptomatic treatment, D – improved clinical findings, E – no clinical finding [8].
Statistical analysis
IBM SPSS Statistics for Windows, Version 21.0 was used for statistical analyses. Data are presented as frequency, percentages, mean ±standard derivation, and median (25th–75th percentile).
Comparisons between groups were analyzed by either the independent samples t-test or the Mann-Whitney U test according to the distribution of normality tested with the Kolmogorov-Smirnov test. The χ2 test was used for the assessment of differences between qualitative variables.
The correlation between disease activity and hematological laboratory parameters was analyzed using Pearson’s correlation test. A p-value < 0.05 was considered statistically significant.
Results
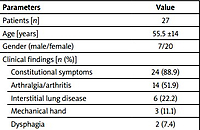
The number of female and male patients was 20 (74.1%) and 7 (25.9%), respectively. The mean age of the patients was 55.5 ±14.0 years (54.0 ±13.0 for females and 60.0 ±16.7 for males). The healthy control group included 30 subjects (17 females and 13 males) with a mean age of 59.1 ±10.1 years (range 35 to 79).
There were no significant differences in age and gender between the patient and control groups. Twenty-four patients (88.9%) had constitutional symptoms, 14 (51.9%) had arthralgia/arthritis, 6 (22.2%) had interstitial lung disease, and 2 (7.4%) had dysphagia. Eighteen and half percent of patients had RF positivity and 66.6% of patients had antinuclear antibody (ANA) positivity.
The mean MITAX and MYOACT scores in patients with polymyositis were 11.3 ±7.9 and 15.9 ±8.8, respectively. Demographic data and clinical characteristics of the patient and control groups are shown in Table I.
Table. I
Demographic data and clinical characteristics of the patient group
Levels of ESR, CRP, CPK, LDH, AST, and ALT were significantly higher in the patient group than in the control group. The platelet count, MPV, PCT, and PDW values were as follows: 352 000 [285 000–403 000], 9.9 ±0.6, 14.6 ±2.1, 0.30 ±0.07, respectively, in the patient group.
The mean MPV and PCT were significantly higher and PDW was significantly lower in patients with polymyositis compared to the control group (p = 0.007, p = 0.003, p < 0.001, respectively). The mean values of platelet count, PCT, MPV, and PDW remained within the normal reference ranges in healthy controls.
The distribution of blood count values of the patient and control group are presented in Table II.
Table II
The distribution of blood count values of the patient and control group
[i] ALT – alanine aminotransferase, ANA – antinuclear antibody, AST – aspartate aminotransferase, CPK – creatine phosphokinase, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, LDH – lactate dehydrogenase, MPV – mean platelet volume, PCT – plateletcrit, PDW – platelet distribution width, RF – rheumatoid factor.
The mean PDW levels were lower in patients with constitutional symptoms (14.4 ±2.2 vs. 16.3 ±0.4, p = 0.04). Also, patients with arthralgia/arthritis had a lower PDW level (12.8 ±2.4 vs. 15.1 ±1.8, p = 0.007).
Although PDW levels were lower in patients with mechanical hand, lung involvement, or dysphagia compared to patients without, there was no statistically significant difference between them. Platelet count was negatively correlated with PCT (p = 0.02, r = 0.568). A negative correlation was found between sedimentation, PCT, MITAX, MYOACT and PDW.
However, no significant correlations were noted between MITAX, MYOACT and platelet count. In addition, we found that ESR was negatively associated with PDW (p = 0.001, r = 0.471; Table III).
Table III
The correlations between disease activity scores, acute phase reactanes and laboratory parameters in patients with ıdiopathic inflammatory myopathies
| Parameters | Platelet | MPV | PDW | PCT |
|---|---|---|---|---|
| ESR [mm/h] | 0.020 | 0.042 | –0.471* | 0.017 |
| CRP [mg/dl] | 0.249 | 0.146 | –0.131 | 0.086 |
| MITAX | 0.163 | 0.003 | –0.619** | 0.305 |
| MYOACT | 0.006 | 0.171 | –0.465* | 0.291 |
Discussion
The myositis disease activity assessment score is used to evaluate disease activity and to predict the course of the disease. It is a better tool than other assessments focusing on muscle damage [9]. Another valid and reliable scoring system, MITAX, measures extramuscular disease activity, and also both scoring systems contain a muscle domain.
The Myositis Intention to Treat Index is a relatively long form and evaluates 6 systems separately: constitutional findings, cutaneous symptoms, musculoskeletal system, gastrointestinal, pulmonary, and cardiac involvement [10].
In our study, the mean scores of MITAX and MYOACT were 11.3 ±7.9 and 15.9 ±8.8, respectively, in the patients with polymyositis. There was also a statistically significant correlation between MITAX, MYOACT and PDW. The International Myositis Assessment Working Group (IMACT) has developed scoring systems consisting of many variables. Creatine phosphokinase and LDH are used in these scoring systems.
Muscle enzymes, especially CPK and myoglobulin, correlate with disease activity. While normal CPK and LDH values do not mean disease inactivation, high serum levels can be detected in patients with clinical improvement. However, it should be kept in mind that they may be an indicator of disease activity and ongoing muscle damage in some patients [11].
It was found that CPK was correlated with the activity of muscle involvement, but was not associated with lung involvement and other systemic findings [12].
In another study, no relationship was found with the MITAX score and other biomarkers (CPK, aldolase, LDH) used in the follow-up. Muscle enzymes such as CPK, LDH, and aldolase are correlated with disease activity, they are used in the follow-up of inflammatory myopathy, but they are not sufficient alone in disease monitoring [13]. The follow-up of CPK and other muscle enzymes will be insufficient in the follow-up of the disease, especially in patients with resistant lung and skin involvement [14].
In our study, the levels of muscle enzymes were statistically significantly higher in the patient group than in the control group. There was no difference in muscle enzymes between patients with and without constitutional symptoms, arthritis, dysphagia, and lung involvement in patients with polymyositis.
Many parameters have been studied to evaluate disease activity in inflammatory myositis. Due to their role in the pathogenesis of inflammatory myopathies, studies have been conducted to evaluate the relationship between disease activation and serum interleukin levels. Interleukin 2 (IL-2) levels were found to be positively correlated with MYOACT and Visual Analogue Scale (VAS) scores [14].
As a result of the disruption of erythropoiesis in inflammation, smaller volumes of erythrocytes are added to the circulation and the RDW value is determined to be higher. In addition, as the neutrophil count increases, the lymphocyte count decreases, and the neutrophil-to-lymphocyte ratio (NLR) increases [15]. Because inflammation and disease activity are correlated in inflammatory myositis, it seems reasonable to use these parameters as biomarkers in detecting disease activation [15].
It was found that white blood cells, neutrophils, NLR, and RDW were significantly higher while lymphocytes, hemoglobin, and platelet count were lower in the patient group (78 with PM and 36 with DM) than in the healthy group.
In another study, the NLR was found to be correlated with the MYOACT score [15].
Platelet parameters such as MPV, PDW, and plateletcrit have been evaluated in many rheumatic diseases. It has been reported that platelet count and MPV values are associated with disease activity and Disease Activity Score 28 (DAS28) in rheumatoid arthritis (RA) [5]. In Sjögren’s syndrome, MPV and platelet count were lower, but plateletcrit was found to be significantly higher compared to healthy individuals [16].
Platelet distribution width is another hematological parameter that shows platelet activation and heterogeneity in platelet size. Systemic inflammation mediated by numerous cytokines stimulates platelet production in the bone marrow [17].
During the inflammatory process in RA, the number of platelets produced by megakaryocytes increases but becomes more monotypic and smaller. As a result, the RDW decreases with identical platelet production. PDW values were reported lower in RA than in healthy control groups [18].
It has been reported that PDW is inversely associated with DAS28, CRP, and ESR, and found to be a negative acute phase reactant in active RA [17]. Patients treated with biological disease-modifying anti-rheumatic drugs (DMARDs) had higher RDW levels than those who did not [17].
In Yu et al.’s [19] study, PDW levels were found to be 0.86 times lower in systemic lupus erythematosus (SLE) patients compared to healthy controls. In this study also a negative correlation was found between PDW and Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), an indicator of SLE disease activity, and low PDW was found to be associated with SLE disease activity.
In another study, higher PDW was found in the SLE patient group compared to the control group, and a positive correlation was found with disease activity [20].
This situation, which was found among studies, may be related to different levels of inflammation in different disease stages. It was highlighted that more studies on larger groups of patients are needed to confirm the prognostic and diagnostic benefits of PDW [19, 20].
There are limited data on PDW in rheumatic diseases, especially in IIMs. The mean MPV and PCT were significantly higher and PDW was significantly lower in patients with polymyositis compared to the control group. Platelet count was significantly negatively correlated with PCT and a negative correlation was found between sedimentation, PCT, MITAX, MYOACT and PDW.
However, no significant correlations were noted between MITAX, MYOACT and platelet count. In addition, we found that ESR was negatively associated with PDW.
Study limitations
The limitation of the study is that the sample size is relatively small since polymyositis is a rare disease compared to other rheumatic diseases.
Conclusions
In conclusion, to our best knowledge, this is the first study assessing the associations between PDW and disease activity in patients with polymyositis.
The present data suggest that PDW levels were lower in patients with a mechanical hand, lung involvement, or dysphagia compared to patients without such symptoms. Also, a negative correlation was found between sedimentation, PCT, MITAX, MYOACT, and PDW.
Therefore, PDW, an inexpensive and easily performed parameter may be useful for a rapid disease activity assessment in polymyositis.