Introduction
Connective tissue diseases (CTDs) are a group of heterogeneous diseases, involving multiple body systems. Among CTDs, rheumatoid arthritis (RA) is one of the most common, with a global prevalence from 0.3 to 1% [1]; less common are systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), systemic sclerosis (SSc), primary Sjögren’s syndrome (pSS) and inflammatory myositis (IM).
There are some biomarkers that are important for the diagnosis and useful to classify CTDs such as: anti-nuclear antibodies (ANA), and other more specific extractable nuclear antibodies (ENA), rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA) and common inflammatory markers: C-reactive protein (CRP), erythrocyte sedimentation rate (ESR). Autoimmune rheumatic diseases are more prevalent among women, e.g. 80–95% of patients with pSS, similarly with SLE and systemic sclerosis [2]; also in ankylosing spondylitis (AS) current epidemiological data showed equal prevalence between females and males [3]. There is a similar situation in other autoimmune diseases such as autoimmune thyroiditis (Hashimoto’s disease) or primary cholangitis [4].
The development of autoimmune rheumatic diseases was linked among other environmental factors (UV radiation, infections) with extensive stress and hormonal imbalance [5].
The basic stressful moment for the human body with hormonal disturbances is pregnancy, which induces various changes in the levels of hormones such as estriol, progesterone and prolactin; also menopause with a decrease of estrogen and progesterone levels is a critical point for the autoimmune system [6, 7]. These two opposite situations represent the influence of hormonal imbalance on the musculoskeletal system [6, 7].
Prolactin (PRL), also known as lactotropin, is a polypeptide hormone, and its chemical structure is similar to the growth hormone (GH) and placental lactogen hormone (PLH). It is responsible for lactation, breast growth [8] and even influences maternal behavior [9]. It was proved that PRL influences lipogenesis and response to insulin in adipocytes [10]. Overproduction of PRL decreases levels of estrogens (female) and androgens (men) [11]. Also PRL has weak gonadotropin and luteotropic effects and has an impact on oligodendrocyte and myelin production and therefore the role of this hormone in the development of sclerosis multiplex (SM) is being studied [12]. Also PRL stimulates neurogenesis in the fetal brain [13].
Prolactin is synthesized by lactotrophs in the anterior pituitary gland that sits directly behind the nasal bridge in a protective boney structure called the “sella turcica” and it is connected to the hypothalamus.
Prolactin production is regulated at the gene transcription level. Factors that stimulate production upregulate prolactin gene transcription while factors that inhibit secretion downregulate prolactin gene transcription. The anterior pituitary gland is the main organ producing PRL, but other organs are capable of producing this hormone, such as mammary glands, the central nervous system, the uterus, and the immune system [11].
In serum this hormone exists in three forms that can be distinguished chromatographically. There are: monomeric PRL (23 kDa), big PRL (45–60 kDa), and big-big PRL or macroprolactin (150–170 kDa). Monomeric PRL constitutes up to 95% of serum PRL, macroprolactin (large antigen-antibody complex PRL-IgG, of molecular weight > 100 kDa) constitutes up to 1% of circulating PRL and can sometimes be a cause of misdiagnosis of hyperprolactinemia [14].
Taking into account the different roles of prolactin and attempts to link the activity of this hormone with the development of autoimmune processes and systemic diseases of the connective tissue, this article attempts to discuss these aspects.
The role of prolactin
Prolactin concentration is dependent on the time of day, the highest concentration being observed at night and the lowest during the day. In women, the physiological concentration of PRL is up to 20 ng/ml, in men up to 15 ng/ml [15]. Prolactin regulates its own release by a short feedback mechanism and acts on prolactin receptors on hypothalamic dopaminergic neurons. A number of factors stimulate or inhibit the secretion of prolactin; they are presented in Table I.
Table I
A pituitary tumor may be the cause of elevated PRL levels. Prolactinoma is the most common type of hormone-producing tumor that can develop in the pituitary gland. It is characterized by proliferation of pituitary lactotrophic cells in the anterior pituitary gland; mostly there are microadenomas (less than 1 cm in diameter) and are asymptomatic. The major effect of a prolactinoma is decreased levels of some sex hormones usually in young females with menstrual irregularities, galactorrhea and infertility.
When PRL levels are increased in females it leads to amenorrhea – absence of menstruation – which results from the prolactin inhibition of gonadotropin-releasing hormone (GnRH) release, while in males PRL level imbalances have different clinical implications and hyperprolactinemia results in headaches and decreased libido [18].
The prolactin receptor (PRLR) is a member of the cytokine receptors that lacks an intrinsic kinase domain but possesses a JAK2-associating region. Prolactin receptor can be activated by three sequence-diverse human hormones – prolactin, GH, and placental lactogen – and being stimulated transduces signals through the activation of JAK2, leading to the phosphorylation of JAK2 [19, 20]. This receptor is composed of three domains: the ligand-binding extracellular domain (ECD), the transmembrane domain and the proline-rich intracellular domain (ICD). Depending on the length of the ICD domain there are different PRLR isoforms – short (SF), medium and long (LF) – and activation of the long isoforms of the receptor leads to cell proliferation while the attachment of prolactin to the short S1a and S1b isoforms inhibits proliferation. The prolactin receptor is found on the surface of most normal human cells, including the liver, epithelial cells of the breast gland, and immune system – monocytes, macrophages, T and B lymphocytes [21].
Its presence is also detected in various types of cancer cells, in which PRL or PRLR affect their progression as in the case of breast, prostate or colorectal cancer, but elevated blood concentrations of PRL have also been reported in patients with pancreatic cancer, and the presence of PRL has also been detected in cancer cell lines of melanoma, leukemia and non-small cell lung cancer [22].
Hyperprolactinemia should also be considered for the presence of big prolactin or big-big prolactin, also known as macroprolactin (over 100 kDa), which is a form of prolactin that is bound to proteins, resulting in an increase in its molecular weight and size. Normally, big and big-big prolactin accounts for about 5–30% of total prolactin in healthy individuals. Macroprolactin is usually biologically inactive and has no prolactin hormone activity. Since laboratory tests are not always able to distinguish between macroprolactin and active prolactin (monomeric), this can lead to falsely high prolactin values in laboratory tests, which can lead to misdiagnosis [23].
Prolactin and connective tissue diseases
It was finally established that PRL may also play a role in the development of SLE, RA and SM [24, 25]. The pathogenesis of SLE is complex and multifactorial and is characterized by abnormal regulation of cellular immunity and deposition of immune complexes. Apart from genetic determinants and environmental factors, hormones play an important role in the development of SLE and the fact is that mainly young women (85%) of childbearing age suffer from lupus especially while pregnant or taking hormonal drugs. Apart from estrogens, PRL may play an important role in the pathogenesis and development of clinical symptoms of SLE [26].
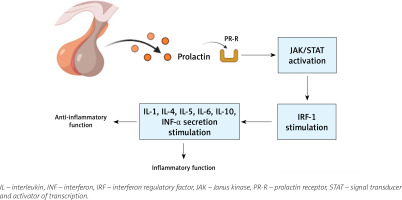
Prolactin can act not only as a hormone but also as a cytokine, being able to modulate immune responses, and hyperprolactinemia may even affect disease activity (Fig. 1). Prolactin regulates the proliferation and survival of lymphoid and myeloid cells, and also affects the selection of T-cell repertoires by influencing the thymic microenvironment [27].
In autoimmune conditions such as SLE, PRL interferes with the activity of regulatory T cells; it also influences B cell tolerance by lowering the activation threshold of anergic B cells. Prolactin promotes production of the CD40 ligand and cytokines such as interleukin-6 (IL-6) and this leads to the production of autoantibodies, one of the hallmarks of SLE. Prolactin enhances the cytotoxic activity of T lymphocytes and the secretion of proinflammatory cytokines [28].
The cytotoxic activity of T lymphocytes and the secretion of proinflammatory cytokines, particularly those belonging to the type 1 interferon (IFN) family, are increased by prolactin, and this is part of the characteristic SLE genetic signature. Prolactin affects neutrophil function and the production of neutrophil traps; it also participates in the maturation and differentiation of dendritic cells and in this way promotes the presentation of autoantigens and high IFN-α secretion, which causes abnormal behavior of both innate and adaptive immune responses.
Prolactin may participate in the process of angiogenesis directly by affecting endothelial cells, or indirectly by affecting the expression of VEGF and other proangiogenic factors, and angiogenesis in synovium is typically observed in RA or psoriasis arthritis (PsA).
Rheumatoid arthritis predominantly affects women, and it has been therefore thought for a long time that hormones such as prolactin and estrogens may play an important role as cofactors in the pathogenesis of this disease. Some studies show that PRLR is expressed by macrophages in RA patients with active disease, and in combination with other inflammatory stimuli relevant to inflammatory arthritis, tends to enhance the expression of multiple chemokines (i.e. CXCL3, 5, 6 and 11) and pro-inflammatory cytokines (IL-6, IL-8, IL-12β) by macrophages.
It has been observed for many years that a decrease of disease activity in RA patients occurs during pregnancy and it is possibly also attributable to an increase of estrogens and progesterone and a period of transient relative hypercortisolism. Later the breastfeeding period might exacerbate disease activity in RA through the release of PRL, and also the risk of developing RA is increased in women who are lactating after their first pregnancy [29].
Tang et al. [30] estimated that synovial PRLR expression is enhanced in patients with inflammatory arthritis compared with osteoarthritis (OA), and even more, that PRL cooperates with other pro-inflammatory stimuli to activate macrophages.
A similar role is attributed to prolactin in the development of psoriatic arthritis (PsA) when elevated vascularization in joint tissue is observed and macrophages are present that are sensitive to the action of prolactin, which intensifies local inflammation. In addition, in the synovial fluid of patients with PsA a high concentration of cytokines such as tumor necrosis factor α (TNF-α), IL-1, IL-6, IL-8 and IL-10 was observed and in conclusion the researchers stated that its synthesis is induced by PRL [31].
Anti-inflammatory role of prolactin
There are theories that prolactin may be immunosuppressive, as in RA, when it improves or goes into remission during pregnancy when the circulating levels of PRL and placental lactogen are high [32], but also it should be noted that its concentration is the highest after childbirth during lactation. Prolactin may inhibit IL-6 gene expression in female reproductive tissues [33].
A protective role may be played by vasoinhibin, a fragment of the PRL that has antiangiogenic and proinflammatory or anti-inflammatory properties. Vasoinhibin signals through receptor binding protein complexes distinct from the PRL receptor and vasoinhibin effects are frequently opposite to those of the full-length hormone [34]. The main role here is played by the PRL/vasoinhibin axis, that is an endocrine axis where the proteolytic cleavage of PRL to vasoinhibin is regulated at the hypothalamus, the pituitary, and the target tissue levels [35]. Vasoinhibin induces the expression of proinflammatory mediators and chemokines in synovial fibroblasts and later matrix metalloproteases and cathepsin D, upregulated in the arthritic joint, cleave PRL to vasoinhibin, and vasoinhibin levels increase in the circulation. The opposite effects of vasoinhibin mean that it is generated during inflammatory arthritis and acts on synovial fibroblasts and endothelial cells to initially promote and later inhibit inflammation, respectively [36].
The pro- and anti-inflammatory role of PRL is presented in Table II.
Table II
The pro- and anti-inflammatory role of PRL [37]
Discussion
There are a lot of data that present clearly the relevance of PRL in autoimmunity, particularly due to its role as a modulator of both innate and adaptive immune responses. When a high prolactin level is found, a big prolactin test is usually performed to determine whether the high prolactin level is really disease related or hyperprolactinemia is due to the presence of big prolactin.
Some results suggest that PRL and PRLR may have potential as new therapeutic targets in inflammatory arthritis. According to other studies there is a difference in the effect of hyperprolactinemia depending on its duration and acute exposure to hyperprolactinemia enhances inflammation during stress, whereas chronic hyperprolactinemia is immunosuppressive [38].
Effects of PRL on the immune response are opposite and essentially associated with PRL concentration, with higher levels being immunosuppressive and lower levels being immunostimulatory [39, 40]. Prolactin stimulates angiogenesis, whereas vasoinhibin inhibits angiogenesis, vasopermeability and vasodilation; however, both PRL and vasoinhibin stimulate the release of vasopressin by the hypothalamo-neuro-hypophyseal system. The influence of the prolactin/vasoinhibin axis in arthritis is suggested by the presence of PRL in the synovial fluid and of PRL, vasoinhibin, and PRL-cleaving matrix metalloproteases in joint tissues including vascular endothelial cells, chondrocytes, fibroblasts, synoviocytes and immune cells.
Hyperprolactinemia promotes the conversion of PRL to vasoinhibin by providing more substrate to cleaving proteases, so its role in the development of inflammation in systemic connective tissue diseases is therefore twofold due to the opposing effects of prolactin and vasoinhibin. However, the role of vasoinhibin in arthritis is more complex, as it has been shown in mouse studies that vasoinhibin also has pro-inflammatory effects and its higher circulating levels coincide with exacerbated arthritis in mice, and vasoinhibin has pro-inflammatory effects in lung tissues [41].
It shows that the role of prolactin in arthritis and connective tissue diseases is double. Hyperprolactinemia is described as a protective factor and there is evidence of a beneficial effect of physiological hyperprolactinemia during pregnancy and after breastfeeding on RA, supported by experimental studies showing that prolonged administration of PRL or genetic deletion of the PRL receptor adequately relieves the course of arthritis, but there is also evidence that it may worsen the severity of inflammatory arthritis. Prolactin signals are found in arthritic joint tissues (chondrocytes and synovial fibroblasts) to inhibit cartilage degradation, synovitis and osteoclastogenesis.
On the other hand, hyperprolactinemia also promotes the conversion of PRL to vasoinhibin, a fragment of PRL that directly stimulates and indirectly inhibits arthritis in a cell type-dependent manner. The role of the PRL/vasoinhibin axis in inflammatory arthritis should still be monitored and further research is needed to help elucidate the role of PRL in rheumatic diseases in order to ultimately develop new therapeutic interventions that can be tested in patients.
However, hyperprolactinemia is not always associated with the consequences of prolactin activity and so in the absence of clinical symptoms indicative of increase of serum PRL concentration may be associated with the presence of macroprolactin. Distinguishing between types of PRL using a properly sensitive laboratory test can be important to avoid misdiagnosis and inappropriate treatment in such a situation [42].
Prolactin level testing should be performed in the case of accompanying other symptoms, such as: menstrual disturbances (irregular or even lack of periods not associated with menopause), infertility, breast milk production when not pregnant or lactating, including in men, erectile dysfunction/impotence and loss of hair on the face and body.
Conclusions
Nowadays, there are no clear indications for routine PRL testing in the diagnosis of systemic connective tissue diseases.
It may also be performed in cases of other pituitary gland diseases, especially tumor with PRL secretion.
The prolactin test can also be used to monitor the treatment of diseases associated with hypersecretion of PRL such as Cushing’s disease and acromegaly, and to monitor the effects of drugs that affect prolactin secretion. In the case of suspicion or confirmation of some systemic connective tissue diseases, it may be useful to test prolactin level as an additional factor influencing the course of the disease.