Janus kinase inhibitors (JAKi) are effective oral agents for rheumatoid arthritis (RA), acting through selective inhibition of the JAK-STAT pathway. Variability in JAK isoform selectivity among agents impacts both efficacy and safety profiles [1]. Emerging data highlight cardiovascular (CV) safety concerns – particularly increased risks of venous thromboembolism, stroke, and ischemic heart disease – when compared to tumor necrosis factor inhibitors, especially in the first treatment year [2]. Given the intrinsically elevated CV risk in RA, thorough risk assessment and management are imperative [3].
Italian guidelines endorse CV risk stratification via the Progetto CUORE algorithm, adjusted by a 1.5 multiplication factor per European Alliance of Associations for Rheumatology (EULAR) recommendations [3, 4]. Based on traditional risk factors, CUORE classifies 10-year CV risk as low, intermediate, or high. However, its performance in RA patients receiving JAKi remains poorly explored [5]. This study evaluates its applicability in a real-world Italian RA cohort on JAKi therapy.
We retrospectively analyzed medical charts of consecutive RA patients fulfilling the 2010 American College of Rheumatology/EULAR criteria [6], treated with JAKi, attending the Rheumatology Unit at the University of Naples Federico II from January 2020 to January 2025. Exclusion criteria included previous CV events. Data collected (Table I) included demographics, smoking status, body mass index, hypertension, diabetes mellitus, lipid profile (total cholesterol, high-density lipoproteins, low-density lipoproteins, triglycerides), and RA-related variables (disease duration, Disease Activity Score 28 based on erythrocyte sedimentation rate, C-reactive protein, Simple Disease Activity Index). Cardiovascular risk was stratified using the CUORE algorithm [8], applying the EULAR-recommended 1.5 multiplication factor. Follow-up data on CV events (myocardial infarction, stroke, venous thromboembolism) were recorded. Informed consent was waived due to the study’s retrospective nature under Italian law.
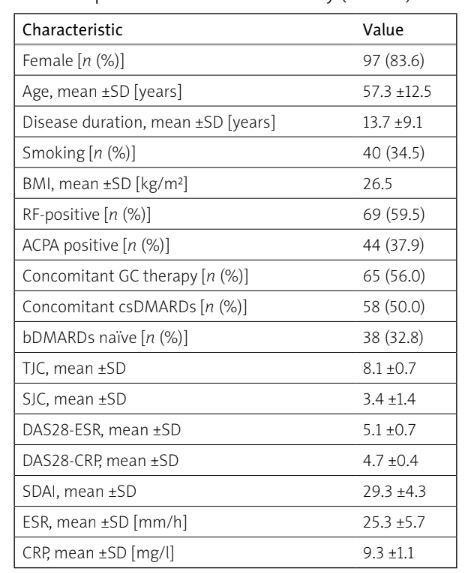
Table I
Baseline demographic and clinical characteristics of RA patients included in the study (n = 100)
[i] ACPA – anti-citrullinated protein antibodies, bDMARDs – biologic disease-modifying antirheumatic drugs, BMI – body mass index, CRP – C-reactive protein, csDMARDs – conventional synthetic disease-modifying antirheumatic drugs, DAS28-CRP – Disease Activity Score 28 based on C-reactive protein level, DAS28-ESR – Disease Activity Score 28 based on erythrocyte sedimentation rate, ESR – erythrocyte sedimentation rate, GC – glucocorticosteroid, RA – rheumatoid arthritis, RF – rheumatoid factor, SJC – swollen joint count, SDAI – Simple Disease Activity Index, TJC – tender joint count.
We enrolled 116 RA patients (83.6% female) with a mean age of 57.3 ±12.5 years. All participants were Caucasian, residing in the Campania region. Median follow-up was 25.6 months (IQR 33; range: 3–60 months). Tofacitinib was used in 24 cases (20.7%), baricitinib in 43 (37%), upadacitinib in 24 (20.7%), and filgotinib in 25 (21.6%).
According to the adjusted CUORE algorithm, patients were stratified into 3 risk categories: low-risk (44 patients, 37.9%), intermediate-risk (56, 48.3%), and high-risk (16, 13.8%).
Over a median follow-up of 25.6 months, only one patient experienced a CV event (myocardial infarction), corresponding to an incidence rate of 0.97 events per 100 patient-years. No cardiac deaths occurred during the study period.
Cardiovascular risk management in RA patients on JAKi remains crucial. Although developed for the general population [4], the CUORE score appears feasible in RA, with low event rates despite many high-risk patients. Given limited prior validation [5], these findings are relevant but should be interpreted cautiously, especially as our cohort predates Food and Drug Administration and the European Medicines Agency safety warnings [7].
Our findings should be interpreted in the context of similar research, such as the study by Cacciapaglia et al. [8], which provided the Italian Society for Rheumatology’s position on CV risk assessment in RA patients. Their multicenter study, offering broader generalizability, found the CUORE score (with EULAR adaptation) to be effective in identifying high-risk patients and underscored the importance of both lifestyle and pharmacological interventions. In contrast, our study, with a smaller, more homogeneous cohort from Campania, found no significant CV events despite identifying high-risk patients. This suggests that factors such as disease duration and medication adherence may influence the predictive value of the CUORE score, calling for a more personalized approach in RA patients on JAKi.
While the CUORE score shows promise, its use in RA patients on JAKi warrants further validation through larger, multicenter studies with extended follow-up. Future research should refine CV risk stratification, integrating disease activity and treatment-related factors to optimize prevention strategies.
This monocentric study, with a small, ethnically homogeneous sample and heterogeneity in follow-up duration, may limit generalizability. Nonetheless, it addresses a key evidence gap, providing real-world data on CUORE score performance in JAKi-treated RA patients.
The EULAR-adapted CUORE score represents a practical tool for CV risk stratification in Italian RA patients on JAKi. Preliminary findings are encouraging, but larger prospective studies are needed to confirm its utility and guide personalized prevention strategies.