Introduction
Behçet’s disease is a systemic vasculitis that presents with various unpredictable symptoms such as recurrent oral and genital aphthae, neurological disease, relapsing uveitis, and other manifestations [1, 2]. The disease can cause blindness, and the therapeutic management focuses on suppressing inflammatory exacerbations and preventing relapses [3, 4].
Severe manifestations require aggressive management with immunosuppressive and biologic agents, alone or in combination with conventional therapies [1, 2, 5]. Some studies have shown the effectiveness of infliximab (IFX), adalimumab (ADA), and rituximab (RTX) for ocular and neurological manifestations of Behçet’s disease [6–10].
However, anti-TNF agents can cause serious side effects such as bacterial infection, viral hepatitis reactivation, hypersensitivity and injection site reactivation, autoimmune disease, and neoplasm [3].
Here, we report our 8-year experience with biologic treatments in Behçet’s disease patients, including the indications, response of each clinical manifestation to treatment, side effects, and reasons for changing biologics.
Material and methods
Study design
This retrospective study analyzed data from patients aged 16–65 years who were diagnosed with Behçet’s disease, met the ICBD criteria [11], and were prescribed a biologic agent for at least 6 months as a treatment for Behçet’s disease.
The patients were referred to the rheumatology clinics of Behçet’s disease of Motahary and Hafez clinics, which are referral centers for Behçet’s disease in southern Iran and are affiliated with Shiraz University of Medical Sciences, from January 2012 to January 2020.
Patients who provided written informed consent on the day of their last visit were included, and those who had no follow-up after 6 months of biologic therapy and could not be contacted by phone or for whom no information was found in other referral centers were excluded.
Assessments
Patient files were reviewed to collect information about the clinical history, management, and reasons for discontinuing or switching biologic treatment. Medication before and during biologic treatment, as well as the disease activity, clinical, biological and radiological response after biologic initiation, time to remission, occurrence of relapse, and side effects before and after treatment were recorded. The review of files was conducted 6 months and 12 months after treatment.
The disease activity on the day of evaluation was assessed by a rheumatologist using a modified Behçet’s Disease Current Activity Form (BDCAF) [12] scoring system, developed by our research team, which included organ involvement over the 4 weeks preceding the assessment according to the original BDCAF, as well as information about the indication for starting biologic treatment and the follow-up assessment of disease activity, partial remission (i.e., improvement), complete remission, relapse rate, biologic-related side effects, poor outcomes, and death, 6 and 12 months after starting biologics. Our scoring system is presented as a supplementary file.
The scoring system assigned one point for each of the following manifestations:
oral ulcers, genital ulcers, skin lesions (such as pustules or erythema nodosum),
joint involvement (arthritis),
gastrointestinal symptoms (abdominal pain or diarrhea with altered/frank blood in rectum),
ocular involvement (confirmed by an ophthalmologist’s examination),
nervous system involvement (confirmed by a neurologist’s examination indicating parenchymal involvement of the brain or aseptic meningitis as neuro-Behçet’s), and
vessel involvement (including arterial or venous thrombosis, aneurysms, or superficial thrombophlebitis).
Only the clinical features related to Behçet’s disease were scored.
Relapse was defined as the reappearance of clinical symptoms and worsening of symptoms. Additionally, event-free survival (EFS) was calculated as the time from the date of biologic treatment initiation to the time of the following events: date of first relapse under treatment or treatment discontinuation after side effect or death (or any other causes) or last follow-up.
Complete remission was defined as disappearance of clinical and paraclinical signs (i.e., disappearance of mucocutaneous manifestations, arthralgia/arthritis and any neurological, digestive or cardiovascular clinical or imaging involvement) using medications along with the prednisolone dose of < 10 mg/day. Additionally, partial remission was defined as any improvement in a specific manifestation compared to its baseline severity.
Other patients were considered as non-responders. Moreover, ocular involvement response to treatment was defined as a decrease to grade 0 in the level of inflammation associated with inactivation of retinal vasculitis and complete resolution of macular edema with a prednisolone dose of < 10 mg/day.
Statistical analysis
Descriptive analysis was carried out using SPSS Statistics (SPSS Statistics Inc., Chicago, US) version 26.0. Results are reported as frequency (percent) and mean (interquartile range [IQR]).
The trend of activity score after starting the biologic agent was analyzed using the repeated measures ANOVA test with pairwise comparisons. The Kaplan-Meier plot was used to perform survival analysis of EFS by the type of biologic agent. A p-level less than 0.05 was considered statistically significant.
Results
We included a total of 44 patients with refractory (93.2%) or severe (6.7%) Behçet’s disease (52.3% female, median age of 34.5 [IQR: 18.5]). The most common inclusion criteria were oral aphthous lesions (97.7%), ocular lesions (75.0%), and genital aphthous lesions (63.6%). In addition, 43.8% and 61.1% of the patients tested positive for HLA B5 and HLA B51, respectively. Table I shows the frequency of each diagnostic criterion.
Table I
Demographic features and frequency of diagnostic criteria (n = 44)
The most common indications for treatment with biologics were ophthalmic activity (68.2%), parenchymal involvement of the brain (15.9%), and joint arthritis (11.4%). Most of the patients (90.9%) were using a biologic agent at the last follow-up, with only a few (9.1%) who had stopped. Infliximab (52.3%) and ADA (45.5%) were the most commonly used first-line biologic agents.
For the second and third biologics, RTX (11.4%), ADA (9.1%), tocilizumab (TCZ) (4.5%), and etanercept (ETC) (2.3%) were used. Only one patient required a third biologic (RTX), and no patient needed to restart a biologic agent. The median cumulative months using the first and second biologic agents were 30 months and 5 months, respectively (Table II).
Table II
Baseline data related to the biologic agents (n = 44)
| Variable | Frequency/out of (%) |
|---|---|
| Usage | |
| Current biologic use | 40/44 (90.9) |
| Biologic ex-usera | 4/44 (9.1) |
| First biologic agent | 44/44 (100) |
| Infliximab | 23 (52.3) |
| Adalimumab | 20 (45.5) |
| Rituximab | 1 (2.3) |
| Second biologic agent | 12/44 (27.3) |
| Adalimumab | 4 (9.1) |
| Rituximab | 5 (11.4) |
| Tocilizumab | 2 (4.5) |
| Etanercept | 1 (2.3) |
| Third biologic agent | 1/44 (2.3) |
| Rituximab | 1 (2.3) |
| Age at starting a biologic agent, median (IQR) | 41 (13.75) |
| Time between diagnosis and TNF-α, months, median (IQR) | 36 (77.75) |
| Indication of starting biologic agent | |
| Ophthalmic activity | 30/44 (68.2) |
| Panuveitis | 19 |
| Panuveitis and retinal vasculitis | 6 |
| Retinal vasculitis/macular edema | 1 |
| Anterior and intermediate uveitis | 1 |
| Only anterior uveitis | 1 |
| Only intermediate uveitis | 1 |
| Nervous system, parenchymal involvement | 7/44 (15.9) |
| Joint arthritis | 5/44 (11.4) |
| Vascular activity | 1/44 (2.3) |
| Genital ulcer | 1/44 (2.3) |
| Gastrointestinal | 0 |
| General indication of starting biologic agent | |
| Refractory Behçet’s disease | 41/44 (93.2) |
| Severe Behçet’s disease | 3/44 (6.7) |
| Need to change biologic agent, with indication | 11/44 (25.0) |
| Refractory Behçet’s disease | 3 |
| Severe Behçet’s disease | 4 |
| Side effectb | 4 |
| Restarting (starting previously used) biologic agent | 0 |
| Duration of using first biologic agent, month, median (IQR) | 30 (20) |
| Duration of using second biologic agent, month, median (IQR) | 5 (29.56) |
Seven patients (15.9%) experienced a side effect with the first biologic agent, mostly hypersensitivity reactions. Of the 12 patients on the second biologic agent, 4 experienced an adverse event (AE), including infections and injection site reactions.
In addition, 3 patients experienced side effects with both first and second biologic agents, including hypersensitivity reaction with IFX (first line) and ETC (second line), hypersensitivity reaction and injection site reaction with IFX (first line) and RTX (second line), and hypersensitivity reaction with IFX (first line) and RTX (second line). Details are shown in Table III.
Table III
Side effects related to biologic agents (n = 44)
| Variable | Frequency/out of (%) |
|---|---|
| First biologic agenta | |
| Negative | 36/43 (83.7) |
| Positive | 7/43 (16.3) |
| Hypersensitivity reaction | 4 |
| Demyelinating disease | 1 |
| Hypersensitivity reaction and injection site reaction | 2 |
| Second biologic agent | |
| Negative | 8/12 (66.7) |
| Positive | 4/12 (33.3) |
| Hypersensitivity reactionb, c | |
| Infectionb | 2 |
| Injection site reactionb | 1 |
Patients showed remission in various symptoms after starting biologic agents, with high rates of remission in oral aphthous lesions (97.7%), ophthalmic activity (93.5%), genital aphthous lesions (96.4%), skin activity (91.7%), arthritis (100%), brain parenchymal lesions (83.3%), and vascular activity (80.0%) at 6 months, which remained unchanged at 12 months.
Similarly, high rates of complete remission were observed for these symptoms at 6 months (ranging from 33.3% to 92.8%), which remained stable or increased at 12 months.
Remission and complete remission were also observed for high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. Gastrointestinal symptoms, deep vein thrombosis, and superficial thrombophlebitis all showed improvement, with complete remission observed for all but the gastrointestinal symptoms case.
Patients with gastrointestinal signs/symptoms, deep vein thrombosis, and superficial thrombophlebitis showed remission at both follow-ups, but complete remission was only observed in cases without gastrointestinal symptoms (Table IV).
Table IV
Remission rates 6 and 12 months after starting biologic agents
All patients were receiving glucocorticosteroid (GC) therapy, but the use of methyl prednisone pulse and high dose oral GC could be stopped during treatment with the biologic agent. Rates of use for colchicine, azathioprine (AZA), and cyclosporine were also changed as well (Table V).
Table V
Frequency of using concomitant immunosuppressants (n = 44)
The median EFS for patients with Behçet’s disease was 2 (IQR = 22.5) months, with poor outcomes observed in 6 (13.6%) patients, including 5 cases of blindness and 1 death. In addition, the mean activity score decreased significantly after 6 and 12 months of biologic agent treatment (F = 179.24, p < 0.0001), with most of the reduction occurring in the first 6 months (mean difference from baseline: at 6-month follow-up = 2.21 [95% CI: 1.79–2.62]; at 12-month follow-up = 2.48 [2.10, 2.85]).
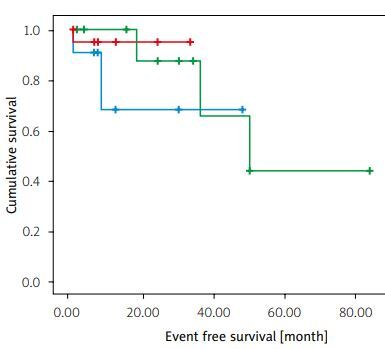
No difference was found in the occurrence of poor outcomes among patients who received IFX, ADA, or a second biologic agent after IFX failure, according to the Kaplan-Meier analysis (log rank χ2 = 0.887, p = 0.642). However, the power of the analysis was low (Fig. 1).
Discussion
The study was conducted retrospectively and evaluated the effectiveness of biologic agents in treating 44 patients with Behçet’s disease, either refractory or severe.
The major finding was that remission was observed in most (range 80.0–100%) of the patients with different types of involvement, including oral aphthous lesions, ophthalmic activity, genital aphthous lesions, skin activity, arthritis, brain parenchymal lesions, and vascular activity, at 6- and 12-month follow-ups. Also, at 12 months, complete remission was observed in some patients with different types of involvement (ranged 33.3–92.8%).
In the study, we found high rates of remission and complete remission in patients with ophthalmic activity. Various studies have also shown significant improvement with IFX and ADA treatments in patients with uveitis and Behçet’s disease [2, 5, 13–17].
In addition, our study found high remission rates for oral and skin lesions at 6-month follow-up. Adeeb et al. [15] also found anti-TNF highly effective against mucocutaneous oral and laryngeal manifestations. Moreover, for less common Behçet’s symptoms, such as arthritis, central nervous system (CNS) parenchymal involvement, and vascular lesions, 80.0–100% of patients had remission at 6 or 12-month follow-up.
Although complete remission was low for CNS parenchymal involvement, this rate was improved for arthritis at the 12-month follow-up. Although there is limited literature to be compared with our results, case-series studies [2, 13] suggest that IFX and ADA can lead to clinical responses in patients with severe and/or refractory joint (70.0%), CNS (91.0–92.3%), and cardiovascular (66.7%) manifestations in Behçet’s disease.
In our study, 25.0% of patients needed to switch to a second biologic agent due to severe disease, side effects, or refractory disease. Anti-biologic agent antibodies can cause secondary inefficacy and there is a need to switch to a second biologic agent [15], but we did not assess them in our study.
Reports show the potential efficacy of RTX, TCZ, and ADA, which are commonly used as second- or third-line biologics, as switched biologic agents in Behçet’s disease. For example, RTX has been used successfully in Behçet’s uveitis refractory to other treatments [10, 18], and TCZ has shown effects in treating multiple symptoms of Behçet’s disease, including ocular, neurological, and vascular involvement as well as secondary amyloidosis [19].
Switching from IFX to ADA has also achieved remission [20]. Another study reported higher rates of switching to a second or third anti-TNF agent but had a longer follow-up than our study.
In consideration of personalizing the selection of biologic agents for Behçet’s disease, we observed high rates of remission across different types of involvement, using various biologic agents, suggesting the potential for tailored therapeutic approaches.
While IFX, ADA, and RTX were the most frequently used biologic agents in our cohort, we could not directly compare them due to the low sample size. However, existing literature highlights the efficacy of specific agents for particular manifestations.
In 2011, Arida et al. [13] performed a pooled analysis of the off-label use of anti-TNF agents in 369 patients with refractory Behçet’s disease from 20 different countries. Since 83% of patients received IFX, there was significantly more information on its therapeutic effect compared with ETC and ADA, making it problematic to compare anti-TNF agents. After 2011, several studies attempted to make this comparison.
Atienza-Mateo et al. [21], studied 177 cases of refractory Behçet’s-related uveitis treated with IFX (n = 103) or ADA (n = 74) as the first-line biologic therapy. They observed favorable results of both therapies after 1 year of follow-up, with ADA showing greater improvement in BCVA and more rapid improvement in anterior chamber inflammation and vitritis.
Fabiani et al. [22] found no difference in efficacy between IFX and ADA for treating non-infectious uveitis. Nonetheless, there is still a need for large-scale randomized controlled trials to establish conclusive evidence for personalized treatment strategies based on individual symptoms and prior treatments in Behçet’s disease.
Our study found no difference in poor outcomes between patients treated with IFX, ADA, or a second biologic agent after IFX failure. This is consistent with a multicenter study by Vallet et al. [2], which found similar efficacy and response rates for IFX and ADA.
Previous research has also shown that switching from a failed first biologic agent to a second biologic agent can be effective, but failure of one anti-TNF drug does not predict a poor response to a second anti-TNF drug [23, 24].
In our study, 16.3% of Behçet’s patients experienced side effects during the first year of biologic agent use. Side effects included hypersensitivity reactions, injection site reactions, and demyelinating disease, all of which can occur after IFX use due to its chimeric nature [21].
Adalimumab had no reported side effects, while RTX had one event each of hypersensitivity reaction, infection, and injection site reaction, and etanercept had one hypersensitivity reaction. Interestingly, three patients who had a side effect with IFX also experienced a side effect with a second biologic agent. Infusion reactions with IFX and skin reactions at the injection site with ADA are the most common side effects [21].
However, biologic agents, such as anti-TNF-α, have been associated with serious side effects, including opportunistic infections [25], latent tuberculosis reactivation [26], demyelinating diseases [27], and malignancy [28, 29]. Longer follow-up periods may be necessary to assess the risk of developing such serious side effects.
In our study, all patients received concomitant immunosuppressive treatment, including GCs, colchicine, azathioprine, and cyclosporine. At the beginning of the study, 31.8% of the patients received high or moderate doses of GCs. However, after one year of biologic agent use, only 11.4% of the patients received a moderate dose, and the remaining patients received a low dose.
Similar to our findings, Arida et al. [13] reported that co-administration of GCs and immunosuppressive drugs with IFX were allowed for tapering or discontinuing GCs in almost all patients. In a Japanese study, IFX monotherapy resulted in a 77.5% GC-sparing effect in Behçet’s-related uveitis over a 10-year period [14].
Another Japanese study showed that IFX use decreased the rates of cyclosporine, GC, and colchicine use in Behçet’s patients, potentially reducing the risk of related side effects [30]. Therefore, biologic agents can efficiently decrease the need for GCs, thereby preventing their side effects, such as secondary cataracts, secondary glaucoma, and diabetes [14].
In our study, 11.4% of patients experienced blindness and 2.3% died, but these events were not caused by biologic agent failure. Rather, these patients had serious pre-existing ophthalmologic damage and poor compliance with biologic treatment.
One of the strengths of the study was the reporting of the effectiveness results for several types of involvement. Similarly to most previous studies in the literature [13–17], ophthalmic activity of Behçet’s disease was the most common reason for starting biologic therapy in this study, followed by CNS parenchymal involvement. In addition, our study had limitations, including a short follow-up period and a small sample size. Additionally, we did not measure anti-biologic agent antibodies.
Due to the differences in the study design, treatment protocols, and patient characteristics, we were unable to compare the effectiveness of different biologic agents. However, we did observe the effectiveness of anti-biologic agents in inducing remission in refractory or severe Behçet’s patients who were unresponsive to conventional immunosuppressive therapy.
Study limitations
Nevertheless, the limitations of our study, including a short follow-up period and a modest sample size, underscore the need for extensive randomized controlled trials to further assess the effectiveness and safety of these agents in Behçet’s disease and compare different biologics and treatment protocols.
Conclusions
Our study suggests that biologic agents can be an effective treatment option for patients with refractory or severe Behçet’s disease, with the potential to induce remission and decrease the need for GCs and other immunosuppressive agents.
Side effects were observed with both first and second biologic agents, but most were not serious. A quarter of patients required switching to a second biologic agent due to severe disease or side effects.