Introduction
Behçet’s disease (BD) is a multisystemic, chronic inflammatory disorder of unknown cause, manifested by recurrent oral and genital ulcers, as well as ocular, vascular, and neurological involvement with cognitive dysfunction [1, 2].
Neurocognitive skills are exclusively confined to the brain and can be strongly influenced by neurological damage and psychological disorders. Competencies that fall under this spectrum include attention, reasoning, mental manipulation, working memory, language, information processing, visual-spatial and perceptual processing, planning, organization and impulse control. It has been demonstrated that neurological and psychological disorders can impact neurocognitive functioning, leading to reduced quality of life and increased disease burden [3].
There are likely to be multi-factorial causes of attenuated neurocognitive functioning in BD and neuro- Behçet’s disease (NBD). The available evidence indicates that both BD and NBD sufferers exhibit higher rates of depression and anxiety than healthy participants and that rates of depression and anxiety in BD and NBD sufferers are similar, with these psychological disorders known to have an impact on neurocognition [4]. Among multiple cognitive domains, memory, visuospatial awareness, attention, and frontal-executive functions were significantly impacted [5]. Those characteristics, however, can develop in patients without neurological involvement, sometimes referred to as “silent NBD” [6–10].
Therefore, it is crucial to expand our knowledge of cognitive performance in BD patients without evident neurological involvement, as the majority of the studies come from patients who have experienced neurological manifestations. The purpose of this study is to determine neurocognitive dysfunction in BD without overt neurological impairment and to identify clinical variables related to cognitive impairment in those patients.
Material and methods
Study population
It was a prospective cross-sectional study that included 40 patients with BD fulfilling the diagnostic International Criteria for Behçet’s Disease (ICBD) without evident neurological manifestations compared with 40 healthy individuals matched for age, sex and education [9]. Patients with BD were appointed from the outpatient clinic of the Rheumatology and Rehabilitation Department, Minia University Hospital. Patients were excluded if they were under 18 years old, had a major psychiatric illness, or if they had a mental disorder or any family history of mental disorders. Patients who had severe ocular involvement were excluded because blindness might interfere with the neuropsychological tests. Patients with metabolic disorders, neoplasms, or serious drug abuse were also excluded from the study. Patients were studied from March 2023 to December 2023.
Clinical evaluation
Each patient was examined clinically by a rheumatologist, neurologically by a neurologist, psychiatrically and psychologically by a psychiatrist and clinical psychologist respectively. The history of each patient revealed no symptoms of neurological involvement and their neurological examination was normal. Psychiatric assessment was carried out based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [11]. Behçet’s disease activity was scored using Behçet Disease Current Activity Form (BDCAF) [12].
Psychometry
Depression and anxiety were further evaluated in both patients and controls using the depression (13 questions), and anxiety (10 questions) subdivisions of the Arabic Version of the Symptom Checklist 90 Revised (SCL-90-R). Every question is ranked from 0 to 4 points, and the total number of points indicates the degree of depression or anxiety [13].
Cognitive function
Both patients and controls were evaluated for cognitive function using a non-computerized neuropsychological battery that was based on validated Arabic Versions of the Wechsler Adult Intelligence Scale-Revised [14] and Wechsler Memory Scale-Revised [15]. The battery took around 60 to 90 minutes to administer and the selected tests did not require language literacy or proficiency as the principles were given to the participants. The assessment covered a range of cognitive domains including both simple and complex attention, memory, visual-spatial processing, language, reasoning, and psychomotor speed [14, 15].
Simple attention and complex attention assessed by Digit Span Forward and Backward respectively
Three to nine digits are presented orally, one group at a time. After hearing a group, participants must repeat it from memory. Some exercises require repetition forward in the same order (Digit Span Forward), others backward in the reverse order (Digit Span Backward). Two trials were administered per digit sequence length. If at least one was correct, the next two trials increased in length [14].
Memory assessed by Wechsler Memory Scale-Revised
The test measures verbal paired associate and paragraph retention, visual memory for designs, orientation, digit span, rote call of the alphabet, and counting backward [15].
Visual-spatial processing evaluated using WAIS-R/III Block Design
Seven designs of increasing complexity must be made using four to sixteen blocks having sides that are white, red, or half white and half red [14].
Language assessed by Vocabulary Scale
Forty-two vocabulary words of increasing difficulty are presented visually and orally. Participants must define each word [14].
Reasoning/problem solving analyzed by WAIS-R/III for Comprehension and Similarities
For comprehension, ten questions ask participants to indicate the correct thing to do under varied circumstances, or why certain practices are followed. For similarities, twelve items require that participants explain the similarity between two things [14].
Psycho-motor speed evaluated by WAIS-R/III Digit Symbol
Nine symbols paired with nine digits are shown. Subjects must then pair the appropriate symbol with the correct digit in a long list of digits. The test is timed. Participants who forget and have to look back at the pairings take longer to complete the test [14].
Patients were classified as having memory deficits if they exhibited abnormal results on one or more tests in the memory domain [15] and cognitive dysfunction was diagnosed if they showed abnormality in two or more cognitive domains [14].
Statistical analysis
Data were analyzed using SPSS Statistics version 20.0 for Windows. Descriptive statistics such as means and standard deviations were used to summarize the characteristics of a data set. The means of quantitative data for more than two groups were compared using Student’s t-test. Pearson’s correlation analysis was used to compute correlations between parameters. Statistical analysis was performed using two-tailed tests and the statistical significance was set at the conventional level of 95%.
Results
Among the 40 studied patients, there were 32 males (80%) and 8 females (20%), male/female ratio 4 : 1. Their age ranged between 19 and 51 years with a mean of 33.8 ±11.2 years vs. 32.65 ±8.43 years in the controls, without any significant difference (p = 0.548). Their age at onset of the disease varied between 12 and 37 years and their disease duration ranged from 6 months to 21 years with a mean of 6.8 ±4.8 years. Mean duration of education in BD and control groups was 11.02 ±3.40 and 12.48 ±3.36 years, respectively, and a significant difference was not observed between the two groups (p = 0.825).
Oral ulceration was the commonest clinical manifestation as it was reported in 35 patients (87.5%), followed by uveitis in 34 patients (85%), genital ulcers in 29 patients (72.5%), skin involvement in 25 patients (62.5%), and arthritis or arthralgia in 19 patients (47.5%). Approximately 23 patients (57.5%) were in active disease assessed by BDCAF.
When the study was conducted, 26 patients (65%) were receiving corticosteroids at a mean dose of 25.25 ±9.7 mg/day, 21 patients (52.5%) were receiving cyclophosphamide, 18 (45%) were taking azathioprine, 11 (27.5%) were on colchicine, 6 (15%) were on adalimumab (15%), 5 patients were on methotrexate (12.5%), 3 (7.5%) were on cyclosporine A, while mycophenolate mofetil was taken by only two patients (5%).
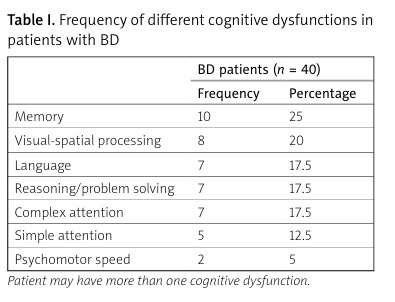
One or more cognitive impairments were identified in 15 patients (37.5%) but for none of the control subjects (p < 0.001). Memory was the commonest affected domain (10 patients, 25%), followed by involvement in visual-spatial processing (8 patients, 20%), then deficit in language, reasoning and problem-solving, and complex attention, which were reported in 7 patients each (17.5%), simple attention deficit in 5 patients (12.5%), and psycho-motor speed deficit was detected in two patients (5%) (Table I).
Table I
Frequency of different cognitive dysfunctions in patients with BD
| BD patients (n = 40) | ||
|---|---|---|
| Frequency | Percentage | |
| Memory | 10 | 25 |
| Visual-spatial processing | 8 | 20 |
| Language | 7 | 17.5 |
| Reasoning/problem solving | 7 | 17.5 |
| Complex attention | 7 | 17.5 |
| Simple attention | 5 | 12.5 |
| Psychomotor speed | 2 | 5 |
The mean SCL-90-R score, for both depression and anxiety, was statistically significantly higher in the patient group than controls (p < 0.05) (Table II).
Table II
Comparison between mean SCL-90-R score for both depression and anxiety in patients and controls
| BD patients (n = 40) | Controls (n = 40) | Difference | ||
|---|---|---|---|---|
| t | p | |||
| SCL-90-R depression | 24.47 ±11.12 | 10.23 ±11.76 | –2.68 | 0.01* |
| SCL-90-R anxiety | 11.47 ±5.45 | 8.23 ±6.34 | –2.71 | 0.01* |
Mean dose of corticosteroid and level of depression measured by SCL-90-R were significantly higher in BD patients with cognitive involvement than patients without cognitive involvement (p < 0.05). There were no statistically significant differences between patients with and without cognitive impairment in terms of age, disease duration, education, disease activity score, or anxiety (Table III).
Table III
Demographic and disease characteristics for BD patients with and without cognitive impairment
| Parameter | BD with cognitive impairment (n = 15) Mean ±SD | BD without cognitive impairment (n = 25) Mean ±SD | Difference | |
|---|---|---|---|---|
| t | p | |||
| Age [years] | 32.14 ±2.97 | 31.36 ±10.00 | –0.66 | 0.51 |
| Disease duration [years] | 6.34 ±2.56 | 5.12 ±1.44 | –0.78 | 0.44 |
| Education [years] | 8.34 ±2.45 | 10.12 ±3.24 | 0.44 | 0.67 |
| BDCAF | 10.81 ±6.23 | 9.56 ±6.13 | –0.27 | 0.80 |
| SCL-90-R for depression | 29.56 ±9.67 | 21.56 ±8.65 | –2.68 | 0.01* |
| SCL-90-R for anxiety | 13.86 ±3.07 | 10.23 ±2.83 | –1.32 | 0.20 |
| Mean dose of corticosteroids (mg/day) | 30.50 ±7.90 | 19.87 ±3.23 | –2.71 | 0.01* |
Table IV displays the correlation between cognitive dysfunction with different disease parameters where cognitive impairment was significantly correlated with current corticosteroid use (r = 0.44, p < 0.01) and depression measured by SCL-90-R (r = 0.41, p < 0.01). However, cognitive involvement was not associated with disease duration, educational years or anxiety.
Table IV
Correlations between cognitive dysfunction and different disease characteristics
| Parameter | Cognitive dysfunction | |
|---|---|---|
| r | p | |
| Age [years] | 0.13 | 0.54 |
| Age at onset [years] | 0.03 | 0.87 |
| Disease duration [years] | 0.18 | 0.33 |
| Education years | 0.34 | 0.06 |
| BDCAF | 0.09 | 0.63 |
| SCL-90-R for depression | 0.41* | 0.01 |
| SCL-90-R for anxiety | 0.06 | 0.73 |
| Glucocorticosteroids | 0.44* | 0.01 |
| Immunosuppressive treatment | 0.12 | 0.55 |
Discussion
Cognitive impairment is a relatively common occurrence in BD, with the majority of data coming from patients with NBD. A review of 74 cases revealed that 87% of BD patients presented some degree of impairment, particularly in memory, attention, and frontal lobe functions, while language and visuospatial skills were relatively preserved [16]. However, studies evaluating cognitive performance in patients with BD patients without neurological involvement are very limited [17].
Our study included 40 Egyptian BD patients who fulfilled the diagnostic ICBD without evident neuropsychiatric symptoms compared with 40 healthy individuals matched for age, sex, and education.
One or several cognitive impairments were identified in 15 of our patients (37.5%) in accordance with a prevalence of cognitive involvement of 40% in a study of 35 Portuguese BD patients without neurological manifestations [5]. In other studies, prevalence of cognitive dysfunction was much higher; according to Monastero et al. [6], cognitive impairment was reported in (12/26) 46.1% of BD patients with normal brain magnetic resonance imaging, as defined by two or more abnormal tests in a standardized battery. Likewise, Urbain et al. [18] reported a prevalence of 47.3%, despite their severe definition of cognitive dysfunction defined by insufficient performance for three or more tests. However, for patients with neurological manifestations, prevalence of cognitive dysfunction was much higher. A higher prevalence of 53.3% was found by Cavaco et al. [5]; the existence of cerebral lesions is a reasonable explanation for the higher level of cognitive involvement reported in that group.
The explanation of this variability may involve the diversity of neuropsychological tests used for assessment whether in traditional or computerized format, language-matched neuropsychological data, and the high degree of variability in the extent to which cognitive testing batteries were applied, from a rather detailed evaluation examining multiple domains [5–8, 19] to studies utilizing one cognitive assessment measure [20]. Moreover, it may reflect the variety of demographic and BD disease characteristics such as age, educational level, disease duration, and disease activity; hence, cognitive function in BD patients deteriorates permanently with time. Another explanation for such variability could be the differences in how cognitive impairment is expressed among different ethnic groups with a variety of outcome definitions of cognitive dysfunction.
In our study, memory impairment was the most affected cognitive domain, accounting for 25% of cases, followed by visual-spatial processing in 20%. This is consistent with prior studies, in which the prevalence of cognitive involvement in BD was 46.1%, with memory and visual-spatial impairment being the most prevalent dysfunctions [6, 21]. Our results also confirm the findings of studies by Urbain et al. [18] and Zayed et al. [22] where memory impairment was the most commonly affected domain in BD patients.
In our study, the effects of corticosteroid usage, age, disease duration, disease activity, depression, and anxiety were assessed in relation to cognitive dysfunction. Only depression and corticosteroid use were statistically significantly correlated with cognitive involvement. In our studied population, BD patients obtained statistically significantly higher scores on depression scales measured by SCL-90-R than healthy individuals. On the other hand, no statistically significant difference was observed regarding anxiety scores between the two groups. This is in agreement with a Turkish study of 30 BD patients comparing 20 healthy controls, whereas the depression score was significantly higher in patients than healthy individuals [21]. Anxiety and depression are both known to affect cognitive functioning; that is why some of the neurocognitive dysfunction observed in BD patients may be related to this [23–25].
Additionally, glucocorticosteroids (GCs) have been shown to have an impact on cognition; in neurocognitive testing, individuals using that medication had a high attribution rate [26, 27]. Glucocorticosteroids can influence a variety of cognitive activities via their effects on both glucocorticoid and mineralocorticoid receptors [28]. The use of corticosteroids in treating BD has made it difficult to differentiate between disease impact and the effect of this type of treatment on cognitive function. Despite this, studies examining the association between GCs and cognitive function in BD yielded conflicting results. In our study, cognitive dysfunction was associated with use of GCs, which was consistent with the findings of Monastero et al. [6]. However, our findings disagree with the study by Cavaco et al. [5], where use of prednisone seemed to protect cognitive function.
Overall, long-term corticosteroid therapy has been shown to increase the rates of psychological disorders [29] and negatively impact cognitive performance [30–32], resulting in a neurocognitive decline pattern defined as “steroid dementia” [33], usually characterized by impairment in declarative memory and concentration [34]. In this context, two possible factors influencing the association between corticosteroid medication and memory were identified in a meta-analysis involving a total of 563 healthy participants. Initially, it was found that giving corticosteroids before learning had no discernible impact on memory; nevertheless, when administered before information recall, a notable mnemonic dysfunction was observed [35], mostly impacting declarative memory retrieval [36, 37]. Secondly, it seems that a significant factor influencing the cognitive effects of GCs is the time of day they are given. More specifically, using small dosages in the afternoon causes a small improvement in memory [38], but taking them in the morning causes memory dysfunction [39], probably as a result of oversaturation of GCs. Furthermore, higher doses of corticosteroids cause memory problems to manifest three to five days after treatment [40, 41]. However, after stopping medication, a quick recovery is observed [42].
One of the main limitations of our study was the lack of a published neuropsychological battery that is universal and standardized for BD, unlike systemic lupus erythematosus, where a standardized neuropsychiatric nomenclature exists, as was reported in the study by Abdel-Nasser et al. [43]. There is a high degree of variation in the size of the cognitive testing batteries applied, from fairly extensive assessment batteries, examining multiple domains, to studies utilizing one cognitive assessment measure. So, there is a need for standardized tests to examine cognitive impairment in BD. Furthermore, longitudinal studies are needed as no data are available on the long-term effects of cognitive dysfunction in BD patients.
Conclusions
Patients who have been diagnosed with BD often experience cognitive dysfunction, particularly affecting memory functions, even if there is no apparent neurological involvement. This impairment is more commonly observed in patients with high levels of depression and those who are receiving GCs. Additionally, patients with BD tend to experience elevated levels of anxiety and depression in comparison to the general population. Therefore, psychological assessment should be performed for every BD patient to reveal any cognitive involvement. It is highly recommended to encourage psychological intervention to prevent any further deterioration, particularly in patients who are experiencing depression or currently using GCs.