Introduction
Despite the wide variety of disease-modifying antirheumatic drugs available for the treatment of patients with various forms of chronic inflammatory arthritis (rheumatoid arthritis [RA] and spondyloarthritis [SpA], including psoriatic arthritis [PsA], often subsumed in the SpA spectrum), the development of new therapeutic approaches and progress in improving patients’ quality of life remain major unmet needs [1]. Individual components of lifestyle, including psychological therapy, exposure to cigarette smoke and smoking, diet optimization, and regular exercise, have been proven to influence the possibility of developing arthritis and constitute a complementary cornerstone of modern treatment [2]. Cumulative data confirm the leading role of nutrition among non-pharmacological treatments in the risk and progression of RA, which is due to the influence of food components on transcription factors, genes, protein expression, synthesis of metabolites, and microbiota [3]. The association of the gut microbiome of SpA patients with dietary factors and disease activity has also been proven. Among foods, red meat, sugar, and sweets have pro-inflammatory properties for RA, while foods rich in vitamins, minerals, and omega-3 fatty acids lead to symptom improvement [4]. Current dietary recommendations for SpA patients are similar to those for RA, including more plant-based foods, vegetables and fruits, and fish, with less meat [5]. The most popular dietary approaches in inflammatory arthritis patients include the Mediterranean diet (MD), vegetarian/vegan diets, elemental/elimination diets, and fasting/calorie restrictions. At the same time, the level of their positive influence differs significantly [6]. Two clinical trials demonstrated significant improvements in grip strength, Ritchie score, and Health Assessment Questionnaire (HAQ) score in RA patients using an elemental diet (protein-free, hypoallergenic diet mainly consisting of glucose, minerals, amino acids, and triglycerides) [7].
Also proven is the positive effect of fasting on the course of RA, PsA, and SpA, both as a separate intervention and in combination with other types of diets, particularly vegetarian [8–10]. A high-quality diet (high intake of fish, fruit, and vegetables, whole grain, and low intake of sausages and sweets) was significantly negatively associated with high sensitive C-reactive protein (hs-CRP) and erythrocyte sedimentation rate (ESR) in RA patients. At the same time, both hs-CRP and ESR decreased with increasing diet quality [11].
There is also knowledge about the impact of certain food substances on arthritis. Lower levels of sodium decrease the inflammatory response by affecting T helper 17 cells in RA patients, and high salt intake is associated with an increased risk of RA [12]. Patients suffering from RA have a higher prevalence of vitamin D (VD) deficiency [13]. Results of studies and meta-analysis demonstrated the impact of VD on RA prevalence, disease activity, and severity of PsA [14, 15]. Vitamin E also potentially benefits RA and PsA management through multiple mechanisms [16, 17]. Positive impacts on RA were also reported for some plants and their compounds [18].
Despite the numerous studies, there are inconsistencies between dietary approaches and RA risk. For example, an association of increased risk of RA with excessive red meat consumption was confirmed in one study [19], but no such effect was found in other studies [20]. Also, data analysis from a large biobank did not reveal a reduction in the risk of RA with a healthy diet [2]. It is also essential to study the eating habits of patients with RA. Thus, several studies have shown that consumption of fruits, whole grains, and plant proteins is lower in RA subjects than in those without arthritis [11, 21]. For SpA, the amount of data on the effect of diet on the risk and course of arthritis is much lower than that for RA, and the available results are primarily from small case-control studies with low or very low-quality scientific evidence; minimal benefits were observed without an apparent effect on objective markers of disease activity [22]. The European Alliance of Associations for Rheumatology (EULAR) taskforce on lifestyle behaviors in rheumatic and musculoskeletal diseases, in its findings on the effect of diet on the progression of rheumatic diseases, identified moderate evidence of a small benefit for specific dietary components and the need for further research in this area [23].
This study aimed to evaluate patient-reported disease activity and fatigue in patients with RA and SpA, including PsA, depending on their dietary preferences.
Material and methods
Study design
Data of patients with RA, SpA/PsA were collected by the Mida Rheuma App [24] (CE-certified mobile application, Midaia GmbH, Germany) from January 2022 to December 2022. Anonymized data of patients were extracted for retrospective analysis. Inclusion criteria were as follows: male or female participants aged 18 and over who have completed disease-specific questionnaires; written informed consent for data sharing and use for research; diagnosis of RA, SpA, PsA, or their combination established by a rheumatologist. After inclusion, the completion of questionnaires assessing the type of diet and the frequency of consumption of certain foods was evaluated, and patients for whom these data were available were included in further analysis. Only baseline parameters were included in the analysis.
Assessments
We assessed demographic parameters (age, gender, diagnosis), major dietary patterns (eat all group [EAG], vegan, vegetarian, MD, and low-carbohydrate diets), and food groups by each patient’s consumption intensity; intolerance to lactose, gluten, fructose, or their combinations was also assessed. Consumption of dairy products, fruits, vegetables, processed meat, meat, oily fish, white fish, and sugar was collected. For each group, participants were asked how often, on average, they had consumed the foods, with 5 responses ranging from 1 “never” to 5 “4 or more times per day/per week” depending on the product. Patients were divided into groups to evaluate the difference in disease activity and fatigue depending on diet, and the high-consumption group (HCG; responses 4 and 5) was compared with the low-consumption group (LCG; responses 1 to 3). Patients on a specific diet were compared with the EAG.
Disease activity was measured by the Patient’s Global Assessment of Disease Activity (PtGADA; scored from 0 to 100 mm), Patient’s Global Assessment of Pain Intensity (PPAIN; scored from 0 to 100 mm), and Routine Assessment of Patient Index Data 3 (RAPID3). Following the RAPID3 score template [25], the score of 0–30 was converted to a score 0–10: a patient who scores between 0 and 1.0 is defined as near remission; 1.3–2.0 as low severity; 2.3–4.0 as moderate severity; and 4.3–10.0 as high severity. Fatigue was assessed by the Brief Fatigue Inventory (BFI; scored from 0 to 10 points) [26].
Statistical analysis
IBM SPSS 22.0 software was used for statistical analysis. Demographic and baseline characteristics were summarized using standard descriptive statistics; mean and standard deviation (SD) were used for continuous variables and numbers and percentages for categorical variables. The data were first checked for a normal distribution using the Kolmogorov-Smirnov normality test. The χ2 test was used to evaluate associations between categorical variables. The differences in the data sets were examined for significance using the t-test. A p-value < 0.05 was considered statistically significant.
Bioethical standards
The study was conducted following the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the appropriate Local Ethics Committee of Medical Center Medical Clinic Blagomed LLC (protocol #107/5, dated 13 Aug 2022). Informed consent was obtained from all patients before the start of Mida Rheuma App use.
Results
Patients
After assessment regarding inclusion and exclusion criteria, data of 1,564 patients were extracted from the database, and 774 completed the “diet type” questionnaire (480 RA; SpA: 180 axial SpA; 61 PsA; 53 – a combination of two types of arthritis). Seventy-nine points eight percent of patients were female, 19.5% male, and 0.7% another gender; the mean age was 42.8 ±12.6 years (Table I). Consumption questionnaires were available for 268 patients. The mean RAPID3, PtGADA, PPAIN, and BFI for patients who completed consumption questionnaires were 3.98 ±2.26, 57.1 ±25.6, 52.9 ±26.4, and 5.39 ±2.23. According to mean values, the average disease activity and fatigue severity were moderate.
Table I
Demographic characteristics
| Group (No. of patients) | Age | Gender | Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female n (%) | Male n (%) | Another gender n (%) | RA n (%) | SpA n (%) | PsA n (%) | Combination of 2 types of arthritis n (%) | |||
| Diet group | |||||||||
| EAG (432) | 43.6 ±13.2 | 331 (76.6) | 70 (22.9) | 2 (0.5) | 276 (63.9) | 99 (22.9) | 30 (6.9) | 27 (6.25) | |
| Vegan/vegetarian (167) | 40.8 ±12.8* | 145 (86.8) | 20 (12.0) | 2 (1.2) | 108 (64.7) | 37 (22.1) | 11 (6.59) | 11 (6.59) | |
| Mediterranean (120) | 42.7 ±12.1 | 94 (78.3) | 26 (21.7) | 0 (0) | 71 (59.2) | 32 (26.7) | 5 (4.17) | 12 (10.0) | |
| Low-carb (55) | 42.4 ±12.2 | 48 (87.3) | 6 (10.1) | 1 (1.8) | 25 (45.5) | 12 (21.8) | 15 (27.3) | 3 (5.55) | |
| Total (774) | 42.8 ±12.6 | 618 (79.8) | 151 (19.5) | 5 (0.7) | 480 (62.0) | 180 (23.3) | 61 (7.9) | 53 (6.85) | |
| Product consumption group | |||||||||
| Dairy | HCG (184) | 44.8 ±12.7 | 136 (73.9) | 47 (25.5) | 1 (0.54) | 108 (58.7) | 44 (23.9) | 22 (12.0) | 10 (5.43) |
| LCG (84) | 43.9 ±13.3 | 65 (77.4) | 18 (21.4) | 1 (1.2) | 55 (65.5) | 17 (20.2) | 9 (10.7) | 3 (3.57) | |
| Meat | HCG (51) | 45.4 ±13.2 | 30 (58.8) | 21 (41.2)# | 0 (0) | 30 (58.8) | 13 (25.5) | 5 (9.80) | 3 (5.88) |
| LCG (217) | 44.1 ±12.4 | 171 (78.7) | 44 (20.3) | 2 (0.92) | 133 (61.3) | 48 (22.1) | 26 (12.0) | 10 (4.61) | |
| Sugar | HCG (65) | 45.1 ±12.7 | 39 (60.0) | 26 (40.0)# | 0 (0) | 40 (61.5) | 14 (21.5) | 7 (10.8) | 4 (6.15) |
| LCG (203) | 44.1 ±13.2 | 162 (79.8) | 39 (19.2) | 2 (0.98) | 123 (60.6) | 47 (23.2) | 24 (11.8) | 9 (4.43) | |
| Processed meat | HCG (58) | 43.5 ±12.0 | 32 (55.2) | 26 (44.8)# | 0 (0) | 33 (56.9) | 16 (27.6) | 6 (10.3) | 3 (5.17) |
| LCG (210) | 44.2 ±13.5 | 169 (80.5) | 41 (19.5) | 2 (0.95) | 130 (61.9) | 45 (21.4) | 25 (11.9) | 10 (4.76) | |
| Oily fish | HCG (176) | 44.9 ±13.1 | 138 (78.4) | 36 (20.5) | 2 (1.13) | 105 (59.7) | 43 (24.4) | 20 (11.4) | 8 (4.55) |
| LCG (92) | 44.0 ±13.3 | 63 (68.5) | 29 (31.5) | 0 (0) | 58 (63.0) | 18 (19.6) | 11 (12.0) | 5 (5.43) | |
| White fish | HCG (113) | 44.1 ±13.1 | 89 (78.8) | 22 (19.5) | 2 (1.77) | 71 (62.8) | 30 (26.5) | 8 (7.08) | 4 (3.54) |
| LCG (155) | 45.3 ±13.0 | 112 (72.3) | 43 (27.7) | 0 (0) | 92 (59.4) | 31 (20.0) | 23 (14.8) | 9 (5.81) | |
| Fruits | HCG (46) | 44.1 ±13.0 | 37 (80.4) | 9 (19.6) | 0 (0) | 20 (43.8) | 8 (17.4) | 14 (30.4) | 4 (8.70) |
| LCG (222) | 45.4 ±12.6 | 164 (73.9) | 56 (25.2) | 2 (0.90) | 143 (64.4) | 53 (23.9) | 17 (7.66) | 9 (4.05) | |
| Vegetables | HCG (63) | 44.4 ±13.3 | 51 (81.0) | 11 (17.4) | 1 (1.59) | 39 (61.9) | 13 (20.6) | 8 (12.7) | 3 (4.76) |
| LCG (205) | 45.3 ±12.7 | 150 (73.1) | 54 (26.3) | 1 (0.49) | 124 (60.5) | 48 (23.4) | 23 (11.2) | 10 (4.88) | |
| Total | 268 | 44.6 ±13.1 | 201 (75.0) | 65 (24.3) | 2 (0.75) | 163 (60.8) | 61 (22.7) | 31 (11.6) | 13 (4.85) |
Disease activity and fatigue in different diet groups
According to diet type, 432 (55.8%) patients were included in the EAG, 21.6% were on a vegan/vegetarian diet (4.8%/16.8%), 15.5% were on an MD, and 7.1% were on a low-carbohydrate diet; 8.4% had lactose, gluten, or fructose intolerance or a combination of them.
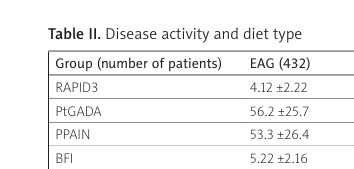
The MD group had statistically significantly lower RAPID3, PtGADA, and PPAIN (by 9.22%, p = 0.047, 8.98%, p = 0.040, and 14.3%, p = 0.007, respectively), compared with the EAG. Vegan/vegetarian and low-carbohydrate diet groups demonstrated only non-significantly better values for PtGADA, PPAIN, and BFI vs. EAG (Table II), with a more significant difference for vegan/vegetarians.
Table II
Disease activity and diet type
| Group (number of patients) | EAG (432) | Vegan/vegetarian (167) | Mediterranean (120) | Low-carb (55) |
|---|---|---|---|---|
| RAPID3 | 4.12 ±2.22 | 4.01 ±2.11 | 3.74 ±2.17* | 4.09 ±2.29 |
| PtGADA | 56.2 ±25.7 | 53.7 ±26.2 | 51.6 ±25.3* | 55.1 ±25.1 |
| PPAIN | 53.3 ±26.4 | 50.2 ±26.7 | 46.6 ±26.1* | 52.8 ±25.7 |
| BFI | 5.22 ±2.16 | 5.01 ±2.23 | 4.95 ±2.20 | 5.16 ±2.14 |
Disease activity and fatigue in different product consumption groups
Statistically significantly higher RAPID3, PtGADA, PPAIN, and BFI were found in the processed meat HCG vs. the LCG: 14.5%, p = 0.034, 18.3%, p = 0.006, 16.1%, p = 0.016, and 11.8%, p = 0.011 for RAPID3, PtGADA, PPAIN, and BFI respectively (Table III). Statistically significantly lower PtGADA, PPAIN, and RAPID3 were found in the oily fish HCG vs. the LCG (18.9%, p < 0.001, 18.1%, p < 0.001, and 10.6%, p = 0.0388, respectively). However, for white fish, this difference was not demonstrated. Although non-significantly better values were found for the vegan/vegetarian group vs. the EAG, the fruit HCG and vegetable HCG showed non-significantly worse values for all outcomes compared with the LCGs. The data in the table support this statement for the fruit HCG but not the vegetable HCG, which has 3 better scores. Non-significantly worse values were also found for dairy products, meat, and sugar consumption, with the most substantial difference for sugar consumption.
Table III
Disease activity and intensity of product consumption
| Consumption group (n/n#) | PtGADA | PPAIN | BFI | RAPID3 | ||||
|---|---|---|---|---|---|---|---|---|
| HCG | LCG | HCG | LCG | HCG | LCG | HCG | LCG | |
| Dairy (184/84) | 56.8 ±27.2 | 53.9 ±23.6 | 55.1 ±27.8 | 52.5 ±26.0 | 5.62 ±2.23 | 5.19 ±2.14 | 4.11 ±2.35 | 3.95 ±2.17 |
| Meat (51/217) | 58.3 ±25.2 | 55.9 ±26.9 | 54.0 ±26.9 | 51.8 ±27.4 | 5.55 ±2.06 | 5.35 ±2.24 | 4.00 ±2.19 | 3.96 ±2.33 |
| Sugar (65/203) | 57.5 ±25.1 | 52.7 ±26.7 | 55.9 ±26.3 | 51.7 ±27.8 | 5.87 ±1.91 | 5.21 ±2.70 | 4.21 ±1.87 | 3.85 ±2.65 |
| Processed meat (58/210) | 61.9 ±24.6* | 52.3 ±27.7 | 56.9 ±24.1* | 49.0 ±26.9 | 5.72 ±1.83* | 5.06 ±2.07 | 4.25 ±2.01* | 3.71 ±1.84 |
| Oily fish (176/92) | 49.4 ±27.7* | 60.9 ±25.3 | 48.1 ±28.4* | 58.7 ±25.0 | 4.85 ±2.21 | 5.11 ±1.73 | 3.78± 2.23 | 4.23 ±1.82 |
| White fish (113/155) | 51.2 ±27.9 | 54.1 ±25.4 | 49.5 ±27.1 | 53.0 ±25.4 | 4.47 ±2.21 | 4.84 ±1.76 | 3.89 ±2.37 | 4.17 ±1.85 |
| Fruits (46/222) | 55.0 ±24.6 | 51.3 ±26.3 | 57.7 ±24.4 | 53.1 ±26.0 | 5.55 ±2.31 | 5.35 ±2.06 | 4.00 ±2.19 | 3.96 ±2.33 |
| Vegetables (63/205) | 52.8 ±27.2 | 54.7 ±28.5 | 51.2 ±26.8 | 54.3 ±29.5 | 4.86 ±1.79 | 5.03 ±1.88 | 4.03 ±2.07 | 3.92 ±2.13 |
Discussion
Our study evaluated the differences in disease activity and fatigue between the groups of inflammatory arthritis patients with different dietary preferences. Detailed data on the amount of individual product consumption made it possible to reveal the differences between disease activity and fatigue indicators not only in groups with different types of diet but also depending on the number of specific products consumed by patients. Among the diets, the MD had the most reliable and statistically significant effect on the course of the disease. In particular, in patients who followed this diet, the self-reported impact of arthritis on their life (PtGADA) and disease activity (RAPID3) were 9% lower, and the pain intensity was 14% lower compared with the EAG. The importance of this dietary approach in patients with arthritis was highlighted in a recent review. In particular, the authors recommended in the conclusions an MD supplemented with at least twice a week consumption of fatty fish and/or n-3 PUFA supplements of 2 g/day as the basis of the diet for patients with RA [27]. Although such conclusions remain controversial in the scope of RA prevention, since the MD affects the risk of RA only in certain groups of patients, the MD reduced the risk of RA in ever-smoking women only in one study [28], and seropositive men in another one [29]. According to other studies’ results, the MD also leads to pain suppression [30] and a decrease of the swollen and tender joint count [31], affecting disease activity indices. Interestingly, the effects of the MD on improving physical function and reducing pain in RA patients, as well as a lower risk of disease, were associated with HLA-DRB1 [28, 29]. The impact of the MD was also evaluated for SpA and PsA. According to the results of a cohort study, low intake of n-3 PUFA and fiber and high intake of ultra-transformed foods were associated with higher SpA activity [32]. The use of 3 g of n-3 PUFA/day for 24 weeks also reduced the need for non-steroidal anti-inflammatory drugs and paracetamol in patients with PsA compared to a control group in which patients received 3 g of olive oil/day. However, the effect on disease activity was not statistically significant for PsA patients in this study [33].
The association of PsA and SpA disease activity with low adherence to the MD was also demonstrated in two studies, which indicates the possible benefit of this diet for these groups of patients [34, 35]. There are several potential mechanisms of the protective effect of the MD in patients with inflammatory arthritis. Modulating effects of the MD on pro-inflammatory genes and oxidative stress [36] and changes in inflammation-related miRNA expression [37] are implemented in reduction of nuclear factor κ-light-chain-enhancer of activated B cells, tumor necrosis factor α, monocyte chemoattractant protein-1 and matrix metalloproteinase-9 expression in peripheral blood mononuclear cells [38], and interleukin-6 (IL-6) and IL-1 levels decrease [39]. The MD shows anti-inflammatory effects also by influencing the gut microbiome [40]. The MD also involves lower consumption of red meat, sweets, and dairy products.
In addition, the phenolic compounds of extra virgin olive oil, one of the essential components of the MD, have beneficial effects on bone by modulating the expression of osteoblast-related genes [41]. In particular, the MD effect is associated with high consumption of fish rich in long-chain n-3 PUFA [42]. Such an influence of this type of fish on disease activity was also confirmed in our study. Patients with a high content of this product in the diet had statistically lower PtGADA, PPAIN, and RAPID3 (18.9%, 18.1%, and 10.6%, respectively) than low eaters. At the same time, there were no differences for white fish.
High fruit and vegetables intake was also associated with low risk of RA, disease activity (Simplified Disease Activity Index – SDAI), functional status (HAQ), and level of matrix metalloproteinase-3 [43, 44]. The probable role of a plant-based diet in decreasing RA symptoms was also demonstrated. At the same time, the effect of such a diet is due mainly to the exclusion of many pro-inflammatory products, the improvement of the composition of intestinal bacteria, and the increase in their diversity [45]. Continuous vegan diet intake improved RA symptoms by decreasing the inflammation in small joints, duration of morning stiffness, and grip strength in a 1-year study [7]. Anti-inflammatory and atheroprotective properties of the vegan, gluten-free diet in RA were also demonstrated by decreasing the low-density lipoprotein and oxidized low-density lipoprotein levels and increasing the level of antibodies against phosphorylcholine [46]. Our study found no significant differences in disease activity or fatigue between the vegan/vegetarian diet group and the EAG, and the HCG and LCG for vegetables and fruits.
Regarding the effect of a low-carbohydrate diet on arthritis, there are only pathogenic speculations about it, but no clinical data confirm this to date. Our study found no differences between the EAG and the low-carbohydrate group [47]. Also, we did not find benefits of other elimination diets, which, according to the authors of a recent review [48], are a powerful tool for reducing inflammation, including due to the effect on the microbiota. However, to date, they are not clearly stated in the guidelines and require predetermining levels of antibodies to dietary proteins.
Among all food groups, a statistically significant negative effect on disease activity was demonstrated only for processed meat and not for meat in general. Patients with high processed meat intake had higher RAPID3, PtGADA, and PPAIN (14.5%, 18.3%, and 16.1%, respectively) than those with low processed meat intake. Patients with high processed meat consumption were the only group that differed in fatigue: high eaters had 11.8% higher BFI than patients who ate less processed meat. It is known that processed meat consumption is associated with an increase of inflammatory mediators, including CRP, and with the risk of RA [49]. But our study is the first to determine the relationship between processed meat consumption and disease activity and fatigue in patients with arthritis. Non-significantly worse scores were also found for dairy products, meat, and sugar, with the most substantial difference for sugar, high consumption of which in seropositive RA women is associated with higher risk of arthritis, independent of other dietary and lifestyle factors [50]. Regarding the effect of meat and dairy products on arthritis, the existing data on the effect of restricting these products on the disease activity relate to small studies [51], and there are currently no convincing data on the negative impact of animal products on the risk of developing or the course of arthritis [52].
Study limitations
The study’s main limitations are the retrospective design and small sample size, leading to bias and influencing the results. The small study population did not allow analysis for each type of arthritis separately, which could help determine individual approaches to diet use depending on arthritis type. However, the data can be used for the general population of patients with inflammatory arthritis since current dietary approaches do not differ significantly. Retrospective analysis caused information bias and data loss, which could affect the study results. Also, the quality of the study was affected by the impossibility of assessing the influence of other factors (for example, differences between groups in drug treatment, smoking status, disease duration, body mass index, physical activity) due to a lack of information in the database and the absence of activity parameters determined by a rheumatologist, which could also affect the results. The limitations mentioned above prevented us from conducting a multivariate analysis to establish the associations between the studied parameters. As a result, the findings we obtained are preliminary and cannot be used to ascertain the impact of dietary factors on the progression of the disease. Nevertheless, the study results can be utilized to identify potential avenues for further research in this field.
Conclusions
Considering the results of our study and comparing them with those of the existing studies, we found that disease activity and fatigue may be associated with dietary factors, potentially leading to less severe disease outcomes reported by patients. The most significant positive influence in our study was demonstrated for the MD and fish rich in long-chain n-3 PUFA, and the most negative for processed meat consumption. Additional research is needed to define the effect of these dietary influences with higher evidence levels.