Introduction
Janus kinase inhibitors (JAKi) are innovative, orally formulated, targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) that have changed the therapeutic landscape in several autoimmune and inflammatory diseases, including rheumatic musculoskeletal diseases (RMDs). With ongoing drug development, both selective and non-selective JAKis have emerged as treatment options in conditions such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) (Table I).
Table I
Janus kinase inhibitors in rheumatic diseases – characteristics
| Name | Receptor selectivity | Therapeutic indications | Eligibility in patients with prior therapy inadequate response or intolerance | ||
|---|---|---|---|---|---|
| Disease, activity and age | Monotherapy eligibility | DMARDs/NSAIDs | One or more TNFi | ||
| Tofacitinib | JAK1, JAK2, JAK3 | Moderate to severe active RA in adult patientsa, b | Preferably in combination with MTXa Monotherapy allowed in cases of intolerance to MTX or when treatment with MTX is inappropriatea | Patient without risk factors: Yesa Nob one or more risk factorsc: Only if no suitable treatment alternatives are availablea, d/Nob | Patient without risk factors: Yesa, b one or more risk factorsc: Only if no suitable treatment alternatives are availablea, d/Yes, when risks and benefits are consideredb |
| Active PsA in adult patientsa, b | Nob | ||||
| Activea AS in adult patientsa, b | Not applicable | ||||
| Active polyarticular JIA juvenile PsA in patients 2 years of age and oldera, b | Yes, in cases of intolerance to MTX or where continued treatment with MTX is inappropriatea | ||||
| Juvenile PsA in patients 2 years of age and oldera | |||||
| Baricitinib | JAK1, JAK2 | Moderate to severe active RA in adult patientsa, b | Yesa Combination with MTX allowed | ||
| Upadactinib | JAK1 | Moderate to severe active RA in adult patientsa, b | Yesa Combination with MTX allowed | ||
| Active PsA in adult patientsa, b | |||||
| Active AS in adult patientsa, b | Not applicable | ||||
| Active nr-axSpa in adult patients with objective signs of inflammationa, b | |||||
| Filgotinib | JAK1 | Moderate to severe active RA in adult patientsa | Yesa Combination with MTX allowed | Patient without risk factors: Yes a, d Patient with one or more risk factorsc: Only if no suitable treatment alternatives are availablea | |
AS – ankylosing spondylitis, DMARDs – disease-modifying drugs, JAK – Janus kinase, JIA – juvenile idiopathic arthritis, MTX – methotrexate, nr-axSpA – non-radiographic axial spondyloarthritis, NSAIDs – non-steroidal anti-inflammatory drugs, PsA – psoriatic arthritis, RA – rheumatoid arthritis, TNFi – tumor necrosis factor inhibitors.
c Risk factors:
- 65 years of age and older,
- with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as diabetes, obesity, hypertension),
- who are current or past long-time smokers,
- current or previous malignancy other than successfully treated non-melanoma skin cancer,
- history of myocardial infarction or heart failure,
- inherited blood clotting disorders,
- history of blood clots,
- combined hormonal contraceptives or hormone replacement therapy,
- major surgery,
- immobility,
- active, chronic or recurrent infections,
- history of serious or opportunistic infection,
- residence or travels in areas of endemic tuberculosis or mycoses.
d For baricitinib and filgotinib a lower dose is recommended for patients with risk factors, with the possibility of a dose escalation in case of inadequate response.
Based on:
Pharmacovigilance Risk Assessment Committee, Assessment report EMA/586384/2022. Available at: https://www.ema.europa.eu/documents/variation-report/xeljanz-h-a20-1517-c-004214-0048-epar-assessment-report_en.pdf [Access 20.07.2023].
Xeljanz: EPAR – Product information. Available at: https://www.ema.europa.eu/documents/product-information/xeljanz-epar-product-information_en.pdf [Access 20.07.2023].
Olumiant: EPAR – Product information. Available at: https://www.ema.europa.eu/documents/product-information/olumiant-epar-product-information_en.pdf [Access 20.07.2023].
Rinvoq: EPAR – Product information. https://www.ema.europa.eu/documents/product-information/rinvoq-epar-product-information_en.pdf [Access 20.07.2023].
Jyseleca: EPAR – Product information. Available at: https://www.ema.europa.eu/documents/product-information/jyseleca-epar-product-information_en.pdf [Access 24.07.2023].
Full prescribing information for XELJANZ/XELJANZ XR/XELJANZ Oral Solution. Revised: December 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf [Access 24.07.2023].
Full prescribing information for OLUMIANT. Revised: June 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf [Access 24.07.2023].
Full prescribing information for RINVOQ. Revised May 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/211675s017lbl.pdf [Access 24.07.2023].
Improvement of disease manifestations within the joints, skin, and gut has established JAKis as an effective form of therapy in RMDs. With longer on-market presence, the safety profile of JAKis has been described as similar to other biologic drugs, though concerns over the excess risk of some adverse events (AEs; infection with herpes zoster virus [HZV], cardiovascular [CV] disease, and malignancy) remain [1].
In 2012, the US Food and Drug Administration (FDA) first approved tofacitinib (TOFA) for the treatment of RA. In subsequent years, indications for the use of TOFA were expanded to include the treatment of PsA, juvenile idiopathic arthritis, AS, and ulcerative colitis (UC) [1, 2].
Due to reported lipid abnormalities, the drug manufacturer committed to a strategy of risk evaluation and mitigation. A large study was designed to assess CV and malignancy risk in patients with RA using TOFA. Two doses (5 mg and 10 mg twice daily) were compared with tumor necrosis factor inhibitor (TNFi) use in patients over 50 years of age, with an inadequate response to methotrexate (MTX) and at least one, concomitant CV risk factor at baseline. All subjects received combination therapy with TOFA and MTX [3].
In 2019, a preliminary study showed an increased, dose-dependent risk of pulmonary embolism for all age groups and increased mortality among patients over 65 years of age, which led to dosage reduction for individuals on 10 mg of TOFA [3].
The European Medicines Agency (EMA) restricted the use TOFA in all indications for patients over 65 years of age, emphasizing caution, and predicating its use on whether alternative therapeutic regimens can be adopted [4]. The FDA recommended TOFA avoidance in patients with enhanced risk of vascular thrombosis. Furthermore, since the 10 mg dose was only utilized in studies involving patients with UC, its use for this indication was limited to cases of inadequate responder status or TNFi intolerance [5].
The ORAL Surveillance yielded several important findings. The primary analysis suggests an increased risk of mortality, major adverse cardiovascular events (MACE), malignancies, and infections for both TOFA groups, regardless of dosing regimen, when compared with TNFi. Notably, efficacy measures were comparable between TOFA and TNFi users [3]. Subsequently, the FDA restricted use of all JAK inhibitors within RMDs, justifying the decision with the potential case of a drug class effect, which warrants particular caution (Table I) [6].
Within the European region, the EMA extended similar recommendations to other JAKis (including filgotinib, which is not FDA-approved), though the regulations are less restrictive and allow the possibility of dose modification as a risk mitigation strategy (Table I) [7].
Real world data from different geographical regions are urgently needed to assist clinicians and policymakers in optimizing RMDs’ management and formal regulation. Our understanding of the JAKi safety profile is still partial, with post-hoc analyses of the ORAL Surveillance report emphasizing the importance of overt CV disease at baseline [8, 9] .
According to some reports, at least one modifiable CV risk factor can be observed in 78.5% of RA patients [10]. Differences regarding the prognostic importance of traditional CV risk factors, as compared with prior history of CV events, also remain unclear. From the patient’s perspective, increased CV risk is placed among the least acceptable AEs in RA therapy [11]. Hence, reporting data from observational studies that investigate these factors may be crucial for future initiatives that will evaluate, pool, and quantify the risks associated with JAKi use.
This retrospective study aims to evaluate TOFA retention and safety using a multicenter cohort of RA patients. Risk factors were evaluated at drug initiation and sequential follow-up was performed to assess drug effectiveness (using composite measures) and safety profile.
Material and methods
This retrospective cohort study was designed to evaluate real world safety and effectiveness of TOFA in the treatment of RA. This study involved five tertiary care centers from different regions of Poland that specialize in the field of rheumatology.
Between June and August 2023, the authors utilized convenience sampling and recruited patients based on electronic medical records obtained from a mandatory innovative treatment registry, supplemented with manual medical chart review (performed by specialists or trainees in the field of rheumatology). Effectiveness and safety data were also supplemented using record linkage with local outpatient clinics. Patients are started on TOFA therapy after failure of at least two conventional DMARD therapies.
Inclusion criteria were defined a priori, as follows: (1) age over 18 years, (2) confirmed diagnosis of RA according to EULAR and American College of Rheumatology (ACR) classification criteria, (3) any TOFA use (any dose) between July 2018 and September 2023. Patients with manifest CV disease were excluded.
The final dataset included information on demographics, arthritis diagnosis, anthropometric measures, treatment schemes, baseline presence of CV risk factors, and detailed information on AEs, as well as composite measures of disease activity. Sequential follow-up was ascertained based on records for visits recorded within the pre-specified monitoring timeframe, in accordance with the drug program, which enables the funding of innovative treatment in Poland. Data were obtained for month 0, 3 and 6, as well as the last available visit (until September 2023).
If applicable, available descriptive information was recorded to further evaluate the causes of treatment discontinuation.
We quantified CV risk using traditional risk factor definitions: smoking status (defined as an ordinal variable; current, ever and never smoking history), age (50 years and older), arterial hypertension, diabetes mellitus, documented history of CV events, dyslipidemia, body mass index (BMI) over 25 kg/m2. Risk factors did not include glucocorticosteroid (GC) dosage, and information on the last available dose was treated as supplementary since we could not reliably calculate the cumulative GC dose. Therapy cessation was categorized based on clinical justification: inadequate response to treatment, occurrence of AE and other causes.
Data were anonymized prior to analysis. We analyzed all records of TOFA initiators for whom a relevant drug initiation date was available, and follow-up information could be reliably extracted (visits recorded for at least 0, 3 and 6 months). Patients were termed: survivors (continuing TOFA therapy until last recorded visit), switchers (in whom treatment was modified due to any cause), responders and non-responders (adequate or inadequate response to therapy as per treatment program guidelines, respectively).
The primary study outcomes were defined as follows:
TOFA retention at 6 months in the total sample,
survival free from AEs necessitating drug switch among responders at 6 months.
Secondary outcomes included:
drug retention and survival free from adverse events (or ineffectiveness) at 12 and 24 months,
incidence of AEs of special interest per 1,000 patient-years: MACE, venous thromboembolism (VTE) or malignancy,
predicted probabilities of drug inefficacy, the impact of CV risk on drug retention and AE occurrence.
Statistical analysis
Statistical analysis was carried out in R 4.3.2 (R Core Team, 2023, R Foundation for Statistical Computing, Vienna, Austria). Continuous and discrete variables are summarized using mean with standard deviation (SD) or count and proportion (n, %), respectively. Missing data were imputed using modal and median values for nominal and continuous variables, respectively.
Variable distribution was inspected visually using density plots and relevant transformation was performed if the distribution was skewed. Comparison across groups was performed using the t test and Fisher’s exact test, for continuous and nominal variables, respectively.
Survival was assessed using crude Kaplan-Meier curves and Cox proportional hazards (PH) models, with the PH assumption tested using Schoenfeld residuals. Tests were two-tailed and a p-value less than 0.05 was considered significant.
Bioethical standards
The study complied with the ethical standards in accordance with the Helsinki Declaration and national regulations in the field. Ethical approval was not required as this was a non-interventional, retrospective database study based on data collected in the course of treatment. Routinely, in accordance with the established rules, all patients were informed about the therapy method and the effects of the drug and then gave informed consent for the treatment. Administrative permissions to process data were granted by all centers.
Results
A total of 223 patients with RA were eligible for study participation and 14 patients were excluded (12 due to the history of a CV event and 2 due to missing data). The final sample consisted of 209 RA subjects. The mean (SD) age of this sample was 51.44 (±11.84) years, and the majority were female (168, 80.4%) (Table II).
Table II
Demographic and clinical characteristics of rheumatoid arthritis patients compared across tofacitinib survivors and switchers
[i] BMI – body mass index, csDMARD – conventional synthetic disease-modifying drug, ESR – erythrocyte sedimentation rate, GC – glucocorticosteroid, HCQ – hydroxychloroquine, LEF – leflunomide, MTX – methotrexate, SD – standard deviation, SSA – sulfasalazine, TOFA – tofacitinib, ts/bDMARD – targeted synthetic or biologic disease-modifying drug.
Dyslipidemia (n = 95, 45.5%), hypertension (n = 51, 24.4%), BMI over 25 kg/m2 (n = 25, 12%), and diabetes mellitus (n = 15, 7.2%) were the most common traditional CV risk factors. Only 30 patients (14.4%) had no pre-existing traditional CV risk factor at TOFA initiation.
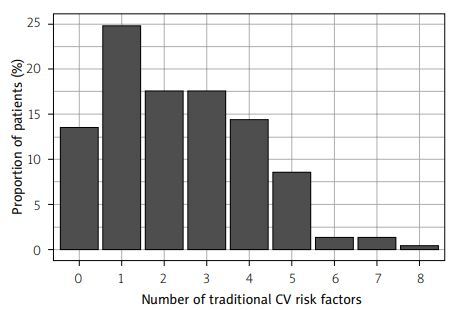
Based on a simple sum of risk factors, we stratified RA patients into low and high CV risk with a cut-off at 3 points: 85 (40.7%) patients with high and 124 (59.3%) patients with low risk. Only 9 (4.3%) patients were maintained on GCs > 7.5 mg/day. Ninety-eight (46.9%) individuals met the simulated ORAL Surveillance criteria of age over 50 years and at least 1 other CV risk factor. The distribution of CV risk (based on a crude risk factor count) is illustrated in Figure 1.
Figure 1
Bar chart comparing the number of traditional cardiovascular risk factors among patients with rheumatoid arthritis treated with tofacitinib.
Most patients used a combination of TOFA and conventional synthetic DMARD (n = 131, 62.7%) and were first-line (n = 100, 47.8%) innovative treatment users (the latter defined as any biologic DMARD or tsDMARD use in a previously naive user). Second- (n = 57, 27.3%) and third- (n = 33, 15.8%) line users were less common, with only 16 (7.7%) and 3 (1.4%) subjects treated in the fourth or fifth line, respectively.
Median (IQR) follow-up in the whole sample was 16.9 (5.93–31.7) months.
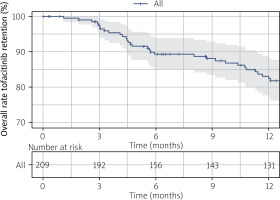
Tofacitinib retention was high in this RA cohort. At 6, 12, and 24 months, median (95% CI) survival was estimated at 89.3% (85%, 93.9%), 82.4% (76.9%, 88.3%), and 60.4% (53.1%, 68.6%), respectively (Fig. 2). We compared clinical characteristics according to retention status (Table II).
Figure 2
Crude Kaplan-Meier survival curve illustrating overall tofacitinib survival among rheumatoid arthritis patients (n = 209).
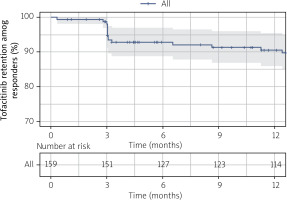
We further examined TOFA retention respective to the incidence of AEs necessitating drug switches among users who were responding to therapy. Estimated median (95% CI) drug survival at 6, 12, and 24 months was 92.8% (88.8%, 97%), 90.6% (86%, 95.4%) and 86.7% (81.1%, 92.8%), respectively (Fig. 3).
Figure 3
Crude Kaplan-Meier survival curve illustrating tofacitinib retention free from AEs necessitating drug switch among responders in rheumatoid arthritis (n = 159).
The reasons for the discontinuation of TOFA during the total follow-up in RA patients were ineffectiveness (n = 50, 23.9%), AE (n = 21, 10%), and other, which included medical (remission) and non-medical factors (e.g. patient’s decision) (n = 3, 1.4%).
At 6 months, there were 126 (60.3%) cases with no record of AEs. One type of AE was observed among 39 (17.7%) patients, while at least two AEs were reported for 46 (22.1%) individuals. Lipid abnormalities (34, 16.3%), infectious events (30, 14.4%), and blood count alterations (29, 13.9%) were the most common within the first 6 months. Due to the timeframe, multiple occurrences were treated as one event per patient.
Patients were followed until the last available visit. Three cases of death were reported: 2 patients suffered from severe COVID-19 pneumonia (one complicated by VTE), and another declined following respiratory failure with concomitant presence of interstitial lung disease (ILD). Additionally, there were other serious AEs: 1 nonfatal COVID-19 infection with concomitant VTE, 1 severe pneumonia, and 1 perforated diverticulitis; AEs of special interest were reported in 3 cases (1 neoplasm (breast cancer), 2 VTE (aforementioned).
Considering a total sum of at least 348 person-years of follow-up in RA patients, the corresponding incidence rate of any AEs necessitating treatment switch was 60.34 (95% CI: 37.35–92.24) per 1,000 person-years of follow-up. Breakdown per specific cause was not performed due to the exceedingly low event rate.
Further, we investigated whether CV burden impacts overall drug retention. In a univariable Cox PH model, the HR for TOFA survival among low CV-risk patients was 1.62 (95% CI: 1.13–2.32; p = 0.008). No relationship was observed between CV risk status and incidence of AEs (necessitating drug switch) among responders with a corresponding HR of 1.41 (95% CI: 0.57–3.52; p = 0.457). The HR for inefficacy was 0.61 (95% CI: 0.35–1.06; p = 0.081) for the subgroup of subjects who did not experience AEs.
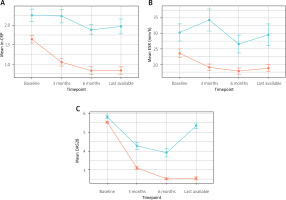
We also considered a simple logistic regression model, observing lower odds of inefficacy tied to low CV risk (OR 0.51, 95% CI: 0.27–0.95; p = 0.034) in the whole cohort. Low CV risk was tied to predicted probabilities of 0.19 (95% CI: 0.13–0.26), as compared with 0.32 (95% CI: 0.23–0.42) in the high CV risk group. Temporal trends in different measures of disease activity are illustrated and compared by effectiveness status (Fig. 4).
Discussion
The main finding of the present report is that TOFA retention was high in a multicenter cohort of RA patients recruited in different tertiary care centers across Poland. Treatment inefficacy was the predominant cause of patient dropout. We also observed relatively low rates of any AEs throughout the early, 6-month treatment period. Follow-up until the last available visit provided a longer timeframe, in which only a few AEs necessitating drug switch were recorded, which supports the current safety measures for TOFA. Furthermore, the presence of multiple traditional CV risk factors was associated with lower TOFA retention and ineffectiveness.
The strength of this study is the real-world character of observation, which precludes the introduction of some sources of bias that exist in carefully controlled clinical trials and allows for more ethical investigation. It is also the first Polish report on the topic. A number of individuals who would likely be excluded from trials (prior history of cancer, sarcoidosis, scleroderma) or even therapy (for a proportion of first-line users, TOFA would not be prescribed according to FDA regulations) were included in this report. We did not observe any trends that would indicate exacerbated risk in these subjects.
To some extent, the retention of TOFA may be higher than in other studies, but lower compared with other European cohorts (86.5% at month 12 and 78.8% at month 24 in an Italian study) [12].
While the study design precludes assessment of causal-effect relationships, these data are important to add to the current state of TOFA safety evaluation. It should be noted that knowing the potential risks tied to TOFA use, patients may be monitored more frequently and managed more intensively. It has been shown that not every risk factor is equal in the context of TOFA use, and the history of atherosclerotic CVD seems to be the most important [13]. In our RA cohort with established CVD cases excluded, higher CV risk still had a negative impact on retention and efficacy.
Adequate lifestyle and treatment modification may improve the added risk attributable to modifiable CV risk factors [14]. As of yet, whether interventions such as statin therapy and consistent attainment of hypertensive therapy goals may reduce any excess TOFA-related CV risk is unclear. Unfortunately, we could not reliably estimate the incidence rate of AEs of special interest, due to the exceeding low count and relatively modest sample size. The main reason for discontinuation was ineffectiveness, which is also in line with earlier reports [12].
The ORAL Surveillance study showed that CV risk may be enhanced due to TOFA use [3]. Similar conclusions can be drawn based on the STAR-RA cohort, which attempted to mimic the ORAL inclusion criteria. In contrast, in the STAR-RA study the incidence of CV events was similar to the TNFi group [15].
In French and Danish cohorts of RA patients, the risk of MACE and VTE for JAKis vs. bDMARDS was observed not to differ significantly [16, 17]. Surprisingly, in some reports, the risk of MACE among JAKi users was even significantly lower than in the TNFi group [18, 19]. In a German study, this observation was applicable even in a high CV risk cohort [20]. In Italian real-world observations, no TOFA discontinuation was caused by a CV event [12], but JAKi users with high CV risk were exposed to a higher risk of AEs [21].
When comparing large randomized trials conducted for TOFA in RA, AS, PsA, and skin psoriasis, they are relatively consistent: the incidence of serious AEs is not higher than in the placebo group [22], with no cases of special interest or even severe AEs [23–25].
In general, higher MACE and VTE risk in TOFA users can be expected in high dosing regimens and in patients with increased baseline CV risk. Although the ORAL study did not show noninferiority of TOFA versus TNFi, it is not unequivocal with cardiotoxicity, particularly when compared with drugs with favorable CV profiles [26, 27]. Inflammation plays a key role in CV risk management [27], and for some patients, ameliorating inflammation may be achieved more effectively via inhibition of the TNF-related signaling axes, while for others, the JAK/STAT pathway may be a more optimal therapeutic target [28].
Study limitations
This study has several limitations. There is a possible selection bias: patients with high CV risk could be prescribed non-JAKi agents.
Aside from the inherent bias associated with the retrospective character, and potential missing information in electronic medical records, data linkage and manual chart review were performed to supplement and enhance the reliability of the present dataset. Moreover, attribution and recall bias may be present as AEs other than laboratory findings were mostly patient-reported.
All patients were treated within the National Health Fund program for innovative treatment, in which TOFA (as well as any other b/tsDMARD) cannot be reintroduced once withdrawn. This restriction may impact the reporting of AEs necessitating drug switch, resulting in hesitation throughout the decision-making process between rheumatologists and patients.
We were also not able to assess other, concomitant CV risk factors, such as cumulative GC dose, individual lifestyle, or physical fitness.
Conclusions
This multicenter, retrospective cohort study evaluated different measures of TOFA effectiveness and safety in RA patients. The strengths of this report include detailed information from a central-eastern European country with low-to-middle income status, and high data reliability due to manual review of medical charts and data linkage with ambulatory care records.
The results of this study indicate a high rate of medium-term drug retention, and a favorable safety profile (with a very low count of serious AEs), even despite the presence of enhanced CV risk recorded within this cohort.