Introduction
Rheumatoid arthritis (RA) is an autoimmune, inflammatory disease with system-wide effects that is related to significant socioeconomic burden and increased risk of mortality, often connected to cardiovascular disease (CVD). The prevalence of atherosclerotic disease is estimated between 30% and 47% [1]. Studies have suggested that the incidence of CV events is independent of traditional risk factor presence, which suggests the importance of other, disease-specific mechanisms [2].
Crowson et al. [3] found that as many as 30% of CV events could be attributed to RA characteristics, i.e. markers of RA severity, regardless of the potential influence of traditional CV risk factors. However, the underlying mechanisms remain incompletely understood, though the influence of heightened systemic inflammation, early development of endothelial dysfunction and lipid alterations is suspected [4].
Recent evidence shows that risk of cardiac death and major adverse cardiovascular events (MACE) is increased when compared with the general population. To illustrate the magnitude, patients with RA have a risk profile comparable to diabetic subjects with regard to risk of myocardial infarct (MI) and percutaneous intervention (PCI) [5].
Studies utilizing ultrasound assessment of carotid intima-media thickness show that progression of atherosclerosis is observed only in individuals with active disease defined as DAS28 > 2.6 [6], which is consistent with the findings of other large prospective cohorts [7]. Even when adjusted for RA-related factors and those of traditional CVD, RA remains significantly associated with post-diagnosis CVD [8].
Taken together, these studies illustrate the importance of controlling disease activity, in conjunction with appropriate management of traditional CV risk factors. The importance of CVD in RA has been recognized by experts, with CVD monitoring recommended every 5 years with appropriate adjustment of population-based risk models [9].
The aim of the present study is to describe the incidence of cardiovascular risk factors in RA patients with low cardiovascular risk at bDMARD initiation.
Material and methods
Study population
In this retrospective study, the authors analyzed 367 electronic medical records (EMRs) of patients with highly active RA [10], screened upon biologic therapy initiation. Consecutive records encoded between January 2001 and June 2017 were considered as the recruitment pool. All patients had a diagnosis of RA according to ACR 1987 [11] or EULAR 2010 [12] criteria.
We excluded 14 patients due to a short follow-up timeframe (< 6 months) and for whom baseline DAS28-ESR could not be calculated. Both inpatient and ambulatory care records were reviewed. The remaining patients were categorized based on pragmatic assessment of CV risk: overt CVD defined as documented coronary artery disease or congestive heart failure; at-risk for CVD if at least one CV risk factors was present (atCVrisk); very low CV risk (vlCVrisk) if no traditional risk factors were present upon initial assessment. Data were obtained using a convenience sample from two tertiary care centers in the region of Lesser Poland.
Follow-up data were gathered between January 2001 and January 2018. Visits were scheduled according to local guideline-based intervals, or clinical indication. The primary outcomes of interest were any newly developed CV risk factors.
Inclusion criteria
The inclusion criteria were as follows:
failure of at least two conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), with each drug maintained for at least 4 months,
highly active disease documented in at least two preceding visits separated by an interval of at least one month; defined as disease activity score 28 (DAS28) using erythrocyte sedimentation rate > 5.1 [10] or simplified disease activity index (SDAI) > 26,
biologic disease-modifying antirheumatic drugs (bDMARD) initiation,
follow-up of at least 6 months following the index date.
The majority (n = 287, 81.3%) of bDMARD initiators were first-time tumor necrosis factor inhibitor (TNFi) users. Anti-IL-6 (n = 39, 11%) and CD20 blocking (n = 27, 7.7%) agent use was less common in first-line bDMARD therapy.
It should be noted that these patients likely represent a different population of bDMARD users when compared with high access nations. Geographical inequities in bDMARD access have been described elsewhere [13], addressing the number of approved and reimbursed bDMARDs and sDMARDs, prices and co-payments, as well as acceptability of bDMARDs (barriers, and the requirement of prior inefficacy of 2 csDMARDs) reflects the current stringency reimbursement criteria in Poland.
Data regarding demographic and clinical characteristics were extracted from the available medical records. Traditional CV risk factors included heart failure (HF), atrial fibrillation (AFib), coronary artery disease (CAD), peripheral artery disease (PAD), diabetes mellitus (DM), hypertension, and lipid disorders. Percutaneous vascular angioplasty procedures were also treated as equivalent to cardiovascular risk.
Major adverse cardiovascular events were further defined as cardiovascular death, central or peripheral revascularization procedure, acute coronary syndrome, cerebrovascular event, or peripheral vascular event. No sourcing of ICD diagnostic codes was considered due to infrequent reporting.
Statistical analysis
Analyses were performed in R 4.3.2 (R Core Team, 2022, Foundation for Statistical Computing, Vienna, Austria) using publicly available packages. Continuous variables are summarized according to distribution, with median (interquartile range – IQR) or mean (standard deviation – SD), as appropriate. Comparison across groups was performed using Fisher’s exact test for categorical variables or the t test/ANOVA for continuous variables. Survival analyses were conducted using unadjusted Kaplan-Meier survival curves with the log-rank test and the Cox proportional hazard model. The proportional hazards assumption was tested. Tests were two-tailed and the p-value was considered significant at p < 0.05.
Results
Incidence of cardiovascular risk factors in patients with rheumatoid arthritis at biologic initiation
For the whole cohort (n = 353), the median follow-up timeframe defined as date of bDMARD initiation and last visit was 41.9 months (IQR 18.6, 80). Overall, 89 (25.2%) individuals developed at least one new cardiovascular risk factor, of whom 65 (18.4%) acquired one, and 24 (6.8%) acquired two or more.
Incident lipid disorders (42, 11.9%), followed by hypertension (14, 4%), atrial fibrillation (17, 4.8%), VTE (16, 4.5%), CAD (9, 2.6%), HF (7, 2%), DM (6, 1.7%) and PAD (2, 0.6%) were observed over the follow-up. Five (1.4%) cases of percutaneous intervention were reported.
For patients presenting with no CV risk factors at biologic initiation (vlCVrisk; “very low CV risk”), newly developed lipid disorders (n = 15, 10.6%), atrial fibrillation (n = 6, 4.2%), VTE (n = 6, 4.2%), hypertension (n = 4, 2.8%), CAD (n = 2, 1.4%) and HF (n = 1, 0.7%) were the most common risk factors. No cases of vascular procedures were recorded.
In contrast, for the at risk group (atCVrisk; “at risk of CVD”), lipid disorders (n = 26, 13.8%), hypertension (n = 10, 5.3%), AFib (n = 10, 5.3%), VTE (n = 10, 5.3%), CAD (n = 6, 3.2%), HF (n = 6, 3.2%), diabetes mellitus (n = 6, 3.2%) and PAD (n = 2, 1.1%) were the most frequently developed conditions carrying CV risk. Four cases of percutaneous intervention procedures were reported.
Comparison of baseline clinical characteristics was performed between three groups of clinical interest (Table I).
Table I
Demographic and clinical characteristics of rheumatoid arthritis patients initiating biologics compared across cardiovascular risk groups
[i] atCVrisk – at least one CV risk factor, bDMARD – disease-modifying antirheumatic drugs, csDMARD – conventional synthetic disease-modifying antirheumatic drugs, CVD – cardiovascular disease, DAS28-ESR – Disease Activity Score with 28-joint count and erythrocyte sedimentation rate, LEF – lymphoid enhancer factor, MTX – methotrexate, SSX – synovial sarcoma X chromosome breakpoint, vlCVrisk –very low CV risk factor. Data missing: BMI for 77 patients, DAS28 baseline value for 4 patients, DAS28 at last visit for 71 patients, serum creatine for 20 patients, total cholesterol for 181 patients, low-density lipoprotein for 189 patients.
Across categories of CV risk, patients did not differ by age or sex, but the highest body mass was observed in patients with manifest CVD. Disease-related factors such as seropositive status, disease activity measures, glucocorticosteroid use or csDMARD choice did not vary between groups. However, parameters of renal function and lipid alterations were highest in patients with CVD or at risk thereof.
Further analysis was also performed across groups stratified by occurrence of new CV risk factors (Table II).
Table II
Demographic and clinical characteristics of rheumatoid arthritis patients initiating biologics stratified by cardiovascular risk and compared according to endpoint status at last follow-up
[i] atCVrisk – at least one CV risk factor, bDMARD – disease-modifying antirheumatic drugs, csDMARD – conventional synthetic disease-modifying antirheumatic drugs, DAS28-ESR – Disease Activity Score with 28-joint count and erythrocyte sedimentation rate, LEF – lymphoid enhancer factor, MTX – methotrexate, SSX – synovial sarcoma X chromosome breakpoint, vlCVrisk – very low CV risk factor.
Incidence of major adverse cardiovascular events among patients with rheumatoid arthritis with no overt cardiovascular risk
Over the course of follow-up (median 42 [21.6–80.6] months), patients with no overt CV disorder had a relatively modest rate of bDMARD retention (36.6% required drug switch due to inefficacy, adverse events or other causes). A third-line biologic agent was reported for 29 (8.2%) patients, of whom 13 (44.8%) developed a new CV risk factor.
No records of incident MACE were reported among patients classified with very low CV risk status at baseline. Conversely, for the at CV risk group, 8 events were reported (5 cases of cardiovascular death, 1 case of acute coronary syndrome, 2 cases of thromboembolic events). The majority of patients who experienced an event were female (n = 7, 87.5%), had seropositive disease (100% aCCP+, RF+), and were treated with maintenance glucocorticosteroids (n = 7, 87.5%). Half required treatment with a second line bDMARD of a different mechanism.
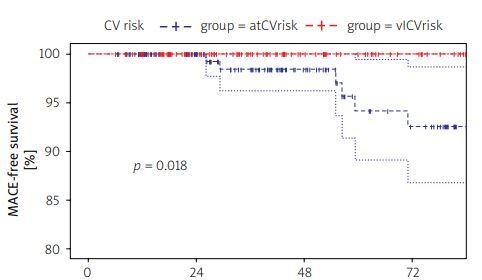
Median survival free from development of MACE was estimated as 0.98 (95% CI: 0.96–1.00) at 36 months and 0.93 (95% CI: 0.87–0.99) at 72 months among patients with no CV disorder at biologic initiation. Figure 1 illustrates differences in MACE-free survival across patients with no overt CVD.
Fig. 1
Differences in major adverse cardiovascular events-free survival across patients with no overt cardiovascular disease.
Survival analyses were performed using the Cox proportional hazards model for patients with no overt CV disorders (both vlCVrisk and atCVrisk groups). Age was a significant predictor of enhanced risk for CV risk factor development (HR = 1.04, 95% CI: 1.02–1.07; p < 0.01). Further analyses were conducted using simple, age-adjusted models.
Only baseline BMI (HR = 1.11, 95% CI: 1.04–1.18; p < 0.01) was an independent predictor of incident CV risk factor status, but not ever smoking (HR = 1.02, 95% CI: 0.63–1.65; p = 0.93), male sex (HR = 1.39, 95% CI: 0.79–2.46; p = 0.26), positive RF (HR = 0.69, 95% CI: 0.37–1.28; p = 0.24), positive autoantibodies against citrullinated antigens (ACPA) (HR = 0.97, 95% CI: 0.61–1.54; p = 0.90), or baseline disease activity (HR = 1.21, 95% CI: 0.91–1.62; p = 0.19).
Discussion
This is a retrospective, longitudinal study that examines the occurrence of CV risk factors in a sample of biologic-initiating patients with highly active RA unresponsive to at least 2 csDMARDs. The median follow-up was over three years. Overall, lipid disorders, hypertension and atrial fibrillation were the most common CV risk factors that occurred.
In patients who had no baseline CV risk factors, further described as low CV risk patients, incident risk factor status was associated with older age and body mass in survival analyses. No CV events were reported in low risk patients over the period of follow-up, which is in contrast to the remaining patients with at least one baseline risk factor (referred to here as “at risk” patients) or overt CV disorders, in both of whom several MACE were recorded.
Large cohort studies have previously demonstrated that when compared with the general population, incidence of hypertension, dyslipidemia and diabetes is significantly higher in RA patients [14, 15]. Lipid abnormalities are among the most common risk factors for CVD worldwide. It has been estimated that over one-third of CV deaths can be attributed to elevated cholesterol levels [16, 17].
In Poland, the prevalence of dyslipidemia is estimated at 77.2%, with 67.1% of adults reported with hypercholesterolemia [17]. At present, lipid disorders were reported as the most common risk factor developed by biologic-naive users, which is in line with the epidemiology of disease described for the general population. As is the case for the majority of cardiometabolic conditions, a bidirectional link between systemic inflammation and CVD likely explains a higher risk for CV risk factor development in RA subjects [9, 18].
We observed that age was a statistically significant predictor associated with CV risk factor development. In population-based studies comparing the rate of CVD in RA patients and controls, a higher rate of close to 30% may be attributed to the presence of RA. Interestingly, while rate ratios for CVD are observed to decline with age, absolute differences increase. This means that although younger patients with RA are at the greatest comparative risk, the absolute number of events increases with age [19].
Age is in itself a unique variable. It can be considered as an indirect measure of the cumulative effect of numerous biologic processes that may contribute to the progression of atherosclerosis. The process of aging has been associated with inflammation and is recognized as an independent risk factor for CVD [20].
On a conceptual level, CV risk factors should be evaluated with respect to an age-related excess risk of CVD. In such an approach, although traditional CV risk factors such as hypertension or diabetes have a considerable contribution to CV risk, age remains the major determinant [21, 22]. Interestingly, in age-adjusted analyses, only body mass appeared to be a significant, independent predictor of CV risk factor occurrence.
Although a causal relationship with CV-related mortality has been extensively investigated in the general population, the effects of some biologic characteristics (e.g., age, body-mass) are difficult and controversial to interpret. It is difficult to differentiate singular effects, rather than those attributed to BMI-associated conditions, which also represent major CV risk factors.
We did not observe an association between smoking status and incident risk factor status. Prior studies have examined the potential association between smoking, ACPA status and disease activity, observing a significant elevation in pro-inflammatory cytokine levels and RA activity among smokers with seropositive status [23].
Smoking has been associated with RA severity in a dose-dependent manner [24], and has also been tied to an increased risk of total mortality and more frequent occurrence of cardiovascular events [25, 26].
Recent studies suggest that RA patients who cease smoking have a lower risk of future CV events [27], though this finding is inconsistent across reports [28, 29]. The lack of association observed for this cohort of RA patients may be derived from interplay between smoking and other, related, concomitant drivers of vascular disease.
Several studies have previously demonstrated a strong association between RA activity and risk of CVD [3, 6, 30–33]. It remains unclear whether specific DMARDs affect an RA individual’s CV risk, aside from the class-specific effects related to abrogation of inflammation. Some evidence suggests that the use of tumor necrosis factor or Janus kinase inhibitors exerts a protective effect against atherosclerosis in RA [34].
Disease-modifying treatment, such as continuous methotrexate use, has been linked to a 20% decrease in incident CV events [35]. Treatment with DMARDs, regardless of type, improves vascular and myocardial abnormalities in RA patients, which highlights the importance of controlling inflammation [36].
In the present study, no significant relationship between baseline disease activity at biologic initiation and new CV risk factor development was observed. However, all patients had highly active disease at baseline, with low variability in DAS28 scores. This makes it difficult to evaluate and quantify the magnitude of risk across the spectrum of disease activity.
Study limitations
Several limitations need to be addressed. This study examined only a modest sample with approximately 1,000 person-years of follow-up, which, in conjunction with low multimorbidity, leads to a low event count.
Second, the data analyzed at present are based on medical records and chart review. Missing information due to potentially incomplete recording is difficult to validate due to the retrospective design. An underlying assumption for the analyses conducted at present is the concept that cardiometabolic disease develops as a byproduct of joint pathophysiologic pathways; therefore nosological entities are grouped under the umbrella term of CV risk factors.
This is a relatively homogeneous cohort of biologic initiating patients, all of whom have highly active disease unresponsive to at least two csDMARDs. In general, the CV burden of this population is low, as no CV risk factors were reported in close to half of patients at drug initiation.
Underdiagnosis of cardiometabolic diseases is an additional problem. Data indicate that RA patients are less likely to receive a diagnosis of, e.g., hypertension, despite experiencing a greater number of provider visits than patients without RA [37]. Taken together, the findings of this study require confirmation in carefully designed, prospective studies.
Conclusions
This report describes patients with highly active RA initiating biologic therapy and evaluates the effects of baseline CV risk profiles on development of future excess CV risk (i.e., new or additional CV risk factors).
The most common risk factors developed over follow-up were lipid disorders, hypertension and atrial fibrillation. Patients with at least one baseline CV risk factor experienced a higher rate of CV events.
While the link between systemic inflammation and CV disease is well recognized, the magnitude of risk in patients with low cardiovascular burden is less understood. The findings of this study suggest that even the presence of one CV risk factor may merit closer follow-up and more stringent disease control among first time biologic users.