Introduction
Several factors should be considered in pharmacotherapy in rheumatology patients with concomitant chronic liver diseases. These include drug toxicity, etiology of the liver disease, severity of liver disease, presence of fatty liver, chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, and other infections [1]. The risk of viral reactivation can also be increased in patients receiving immunosuppressive and biologic treatments based on the type and duration of these medications, the levels of liver enzymes, and the status of HBV infection (serum HBV DNA level) and the severity of the liver disease. The risk of HBV reactivation (HBVr) is divided into low (< 1%), moderate (1–10%), and high (> 10%) [2]. For example, among the most important high-risk drugs are rituximab (RTX) and high-dose glucocorticosteroid (GC; prednisolone ≥ 20 mg/day for ≥ 4 weeks); moderate-risk drugs include tumor necrosis factor inhibitors (TNFi); and low-risk drugs include azathioprine (AZA), methotrexate (MTX) and any dose of oral GCs for ≤ 1 week or low dose (< 10 mg daily) for ≥ 4 weeks [3]. All patients undergoing treatment with a biologic or immunosuppressant with high or moderate risk of HBVr should be subjected to screening (serum evaluation of HBsAg, anti-HBc, and anti-HBs). The prevalence of chronic liver disease and cirrhosis is increasing worldwide, especially metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease [4, 5]. Metabolic dysfunction-associated steatotic liver disease has been reported in 25% of the general population, 30% of patients with rheumatoid arthritis (RA), and in an even higher percentage of patients with psoriatic arthritis [6].
Liver metabolism accounts for the elimination of many drugs. The liver has a high functional reserve, and a significant hepatic impairment must occur before the metabolism of the drug is affected. There is, however, no readily available marker to estimate the degree of hepatic impairment and guide drug dose adjustment in liver disease. There are multiple interacting factors in patients with liver cirrhosis including impaired hepatic metabolism of drugs, liver blood flow, binding to plasma proteins, and biliary excretions, which may require adjustment of the dosage or even avoidance of some medications to reduce the risk of toxicity [7]. Patients with severe liver diseases may have impaired elimination or activation of drugs. In addition, decreased albumin may lead to an increased free fraction of some active components of drugs [8].
Severe liver disease is defined when the synthetic function of the liver is compromised and there is clinical evidence of decompensation. Advanced liver disease or cirrhosis is documented by liver pathology or non-invasive tests. Elevated transaminases greater than 3 times the upper limit of normal should also be a concern. Also, renal function can be overestimated in patients with advanced liver diseases, and this should always be considered before prescribing medication [8]. Different classification systems have been used to grade the severity of liver disease in patients with cirrhosis, amongst which the Child-Pugh classification and Model for End-stage Liver Disease (MELD) score are the most commonly used scoring systems [9].
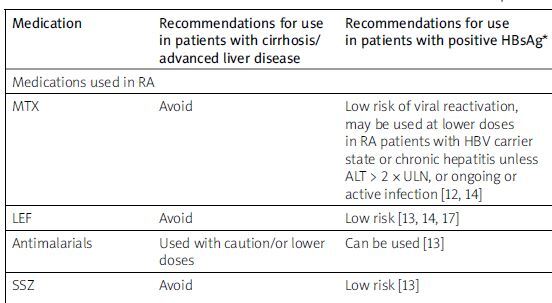
Herein, we will discuss common considerations in prescribing antirheumatic drugs in patients with chronic liver disease including dosage adjustment, contraindications, and precautions in most common rheumatologic diseases based on a search on PubMed using key words of the most used rheumatologic medications, advanced liver diseases, MASLD and viral hepatitis from 1980 to 2024. In cases of contradictions in guidelines or findings, the most recent ones were included, and previous ones were excluded. We also used drug manufacturer recommendations as well as rheumatology and pharmacology books to create a collection of pharmacological consideration of importance in pharmacotherapy of patients with rheumatologic patients. The summary recommendation and dosing adjustments for biologic and immunosuppressant agents in patients with cirrhosis/ advanced liver disease, chronic and resolved hepatitis B, and in hepatitis C are shown in Table I.
Table I
Summary recommendations and dosing adjustments for biologic and immunosuppressant agents in patients with cirrhosis/advanced liver disease and chronic and resolved hepatitis B
| Medication | Recommendations for use in patients with cirrhosis/advanced liver disease | Recommendations for use in patients with positive HBsAg* | Recommendations for use in HBsAg-negative but HBcAb-positive (resolved HBV infection)* |
|---|---|---|---|
| Medications used in RA | |||
| MTX | Avoid | Low risk of viral reactivation, may be used at lower doses in RA patients with HBV carrier state or chronic hepatitis unless ALT > 2 × ULN, or ongoing or active infection [12, 14] | Rare [12], relatively safe |
| LEF | Avoid | Low risk [13, 14, 17] | Relatively safe |
| Antimalarials | Used with caution/or lower doses | Can be used [13] | Can be used [13] |
| SSZ | Avoid | Low risk [13] | Low risk [13], relatively safe, one case report of de novo hepatitis activation in a patient with prior HBV infection |
| TNFi | No dose adjustment, high prevalence of serious infections, should be avoided in decompensated cirrhosis | High risk [43], moderate [12], antiviral therapy should be initiated 1 week prior to starting treatment with RTX and should continue for at least 12 months after stopping | Low risk of HBV reactivation (0–8.3%) [12], moderate risk for anti-TNF agents with higher potency: adalimumab, infliximab, golimumab, certolizumab, low risk for anti-TNF agents with lower potency: etanercept [2] |
| RTX | No dose adjustment needed | Very high risk of HBV reactivation (10–20%) [2] | |
| TCZ | Avoid | Moderate to very high risk of HBV reactivation in different literature (1–60% chance) [37] | Low (1.6%) risk [13, 14] |
| JAKi | Avoid | High risk of viral reactivation [14] | High risk [12, 43] |
| Ixekizumab | No dose adjustment | Moderate to high risk of increased HBV reactivation [16] | Low risk [63] |
| ABT | No need for dose adjustment | Medium risk [14], medium to high risk [13] | Low risk [14] |
| Prednisone | No need for dose adjustment | High risk (> 10 mg, > 4 week), medium risk (< 10 mg, > 4 week), low risk (< 10 mg, < 1 week) [14] High risk of viral reactivation for high dose ≥ 20 mg/day for ≥ 4 weeks Moderate risk of viral reactivation for median dose: 10–20 mg/day for ≥ 4 weeks Low risk of viral reactivation for low dose < 10 mg/day [2] Intra-articular < 1% [13] | Low risk of viral reactivation for high dose (≥ 20 mg/day), intra-articular < 1% [13] |
| Medications used in SLE | |||
| AZA | Use with caution, no dose adjustment | Low risk of viral reactivation [2, 12, 13] | Low risk, rare [12] |
| CP | Avoid | Moderate risk [2], high risk [13] | Low risk, rare [12] |
| CsA and TAC | Avoid | High [12], moderate [13] | Low [13], moderate [12, 16] |
| Mycophenolate mofetil | No dose adjustment (no more than 2 g/day) | Low risk [13] | Low [13] |
| Belimumab | No dose adjustment | No study | No study |
| Medications used in spondyloarthropathies | |||
| Secukinumab | No dose adjustment | Moderate risk [3] | Very low [63] |
| Apremilast | Safe, no dose adjustment | Low risk [63] | Safe [63] |
| Ustekinumab | No dose adjustment | Moderate risk [2, 3], high risk [13] | Moderate risk [13] |
* It is recommended that in the case of high- and moderate-risk medications in HBsAg-positive patients and high-risk medications in HBsAg-negative but anti-HBc-positive patients, potent nucleos(t)ide analogues should be initiated at least 1 week (ideally 2 weeks) before starting them [14]. For those with moderate risk, all HBsAg positive and those HBsAg-negative but anti-HBc-positive patients with advanced liver fibrosis or cirrhosis should be administered potent nucleos(t)ide analogues. For HBsAg-negative and anti-HBc-positive patients without advanced fibrosis or cirrhosis, serum ALT should be monitored every 3 months. If elevated ALT > 2 × baseline is detected at monitoring, HBsAg and HBV DNA should be performed and potent nucleos(t)ide analogues initiated if either test positive. For those with low risk, potent nucleos(t)ide analogues should be initiated in both HBsAg positive and HBsAg-negative but anti-HBc-positive patients with advanced fibrosis or cirrhosis. Serum ALT should be monitored every 3 months in both HBsAg-positive and HBsAg-negative but antiHBc-positive patients with low-risk. Antiviral treatment should be continued for at least 6 months after discontinuation of biologics and immunosuppressants and at least 12 months for B cell-depleting agents [2]. The most frequently used potent nucleos(t)ide analogues are lamivudine, entecavir, adefovir, tenofovir disoproxil fumarate, and tenofovir alafenamide. Entecavir has the greatest efficacy in prophylactic treatment [60].
ABT – abatacept, AZA – azathioprine, CP – cyclophosphamide, CsA – cyclosporine A, JAKi – Janus kinase inhibitors, LEF – leflunomide, MTX – methotrexate, RTX – rituximab, SLE – systemic lupus erythematosus, SSZ – sulfasalazine, TAC – tacrolimus, TCZ – tocilizumab, TNFi – tumor necrosis factor α inhibitors.
Medications used in rheumatoid arthritis
Methotrexate
Methotrexate is excreted in the bile and undergoes enterohepatic circulation; therefore, it should be avoided in patients with impaired biliary function [8]. It should also be avoided in patients with severe hepatic impairment (alanine transaminase [ALT] > 2 × ULN, ongoing or active infection, and advanced liver disease due to chronic hepatitis B or C), which can increase the risk of MTX toxicity [10].
The 2021 American College of Rheumatology (ACR) guideline for the treatment of RA recommended MTX over alternative disease-modifying antirheumatic drugs (DMARDs) in patients with MASLD when liver enzymes and liver function tests are normal and there is no evidence of advanced liver fibrosis (stage 3 or 4). Also, if the medication is started, patients will need more frequent monitoring of liver tests (every 4 to 8 weeks) [11].
Methotrexate is relatively safe in RA patients with chronic or resolved HBV infection, with low risk of HBVr [12]. However, low rates and rare cases of reactivation have been reported in a few large cohort studies. There was a report on 18 cases of HBVr in 768 HBcAb-positive rheumatic patients using MTX alone or combined with other drugs, and low risk of viral reactivation was indicated in the labeling, but it may increase in combination with GCs or other immunosuppressive agents [13].
The use of long-term MTX has not been significantly associated with an increased risk of liver cirrhosis among RA patients with chronic hepatitis B. Methotrexate may also be used at lower doses in RA patients with HBV carrier state [14, 15].
In addition, long-term MTX use has not been associated with an increased risk of liver cirrhosis among RA patients with chronic hepatitis C [15]. A few studies have evaluated MTX in patients with positive HCV, and in 39 cases, only one reactivation has been reported [16]. However, the results should be interpreted with caution due to the potential bias in the cohort studies resulting from the exclusion of patients with more severe forms of liver diseases.
Leflunomide
Leflunomide (LEF) is excreted in the bile and undergoes enterohepatic circulation; therefore, it should be avoided in patients with impaired biliary excretion. It should not be used in patients with severe hepatic impairment (ALT > 2 × ULN), chronic hepatitis B or C infection, and evidence of hepatic dysfunction [7]. Leflunomide is a prodrug and needs to be converted to an active form by the hepatic metabolism. Severe liver disease may impair the metabolism of LEF [8]. In an in vitro study, it was found that it could directly upregulate HBVr and expression [13]. In patients with hepatitis B infection (HBsAg-positive), LEF may activate HBVr and may cause liver damage. Cases related to LEF-induced HBVr are rarely reported, although, in 36 patients with HBsAg-negative, HBeAb, and/or HBcAb-positive RA, there was no HBVr after 6 months of a low dose of LEF treatment [13]. Thus, it is contraindicated in such patients [14]. In another study on patients with RA which used a low dose of LEF, the multiple regression model showed a 30% risk of HBVr [17]. Based on a study of 36 patients with RA carrying HBV, 4 of 5 patients (80%) treated with MTX + LEF and 21% (6/28) of patients receiving MTX alone or combined with other DMARDs developed hepatitis reactivation and it was considered high risk [14]. In most articles to date, LEF is regarded as a low-risk drug, which requires monitoring only. Based on all previous studies, it is considered a low-risk drug for HBVr [13].
Antimalarials
Antimalarial compounds should be used with caution in patients with hepatic disease or alcoholism, as the manufacturer recommended. Neither chloroquine (CQ) nor hydroxychloroquine (HCQ) is associated with a significant risk of liver enzyme elevation or hepatotoxicity. They have not been associated with ALT abnormalities, so they are the commonest drugs used in the majority of studies in RA patients with cirrhosis [12]. They can concentrate in the liver and should be used with caution or in lower doses in patients with hepatic impairment and those with alcohol use disorders [8, 18]. During the coronavirus disease 2019 (COVID-19) pandemic with increased use of antimalarial drugs for the treatment of COVID-19, no significant liver-related adverse events related to the use of these medications were reported [18]. Since they do not need liver enzyme monitoring during treatment, the ACR recommends that they can be good treatment options for RA patients with hepatitis C, which may or may not need antiviral treatment [19]. The safety of antimalarial medications in patients with hepatic impairment has not been studied yet; however, Firestein & Kelley’s Textbook of Rheumatology stated that antimalarial drugs are contraindicated in severe liver disease caused by viral hepatitis [10, 18]. Hydroxychloroquine may down-regulate TNF-α, leading to HBVr. Although cases of HBVr are extremely rare, HCQ is generally considered safe in patients with chronic viral hepatitis [13].
Sulfasalazine
It is recommended to avoid sulfasalazine (SSZ) in severe liver disease and active viral hepatitis [10, 20]. It has received little attention regarding HBVr in patients with rheumatic diseases, although there is one case report of de novo hepatitis activation after SSZ monotherapy in a patient with RA with prior HBV infection (HBsAg-negative, HBsAb, and HBcAb-positive), due to polymorphisms of ABCG2 and NAT2, which could lead to high plasma concentrations of sulphapyridine and may lead to reactivation of hepatitis B infection [21]. In a study done on 39 RA patients with persistent HBsAg positivity, SSZ, HCQ or a combination of them was used for about 12–16 months; SSZ seemed to activate latent virus B hepatitis more than the other groups [22].
Tumor necrosis factor-α inhibitors
Clinical trials have shown that the use of TNFi in patients with severe acute alcoholic hepatitis and decompensated cirrhosis as well as those with autoimmune hepatitis has been associated with a high prevalence of serious infections. It was recommended that TNFi might be used in cases of MASLD without severe fibrosis, by using screening with non-invasive methods (transient elastography or biomarkers). However, in the case of severe fibrosis, data are equivocal. Tumor necrosis factor inhibitors should be avoided in decompensated cirrhosis of any cause, because of the increased risk of potentially serious infections [16]. In compensated MASLD-related cirrhosis, TNFi must be used with caution, paying particular attention to the infectious risk [6]. In a case series of 23 patients diagnosed with psoriasis and cirrhosis (alcoholic or mixed alcoholic/non-alcoholic steatohepatosis mostly with mild, scoring as Child-Pugh A disease) before beginning biologic therapy, the patients received a TNFi with a median duration of 31 months. Five patients experienced 6 adverse events during treatment, including erysipelas of the leg and non-severe bacterial pneumonia; it was recommended that biological therapies could be a good option in the treatment of severe psoriasis in patients with cirrhosis. However, extreme caution should be taken [23].
The association between TNFi use and increased HBVr has been well established. The risk of increased HBVr is moderate to high (9.1–75%) in HBsAg-positive RA patients without antiviral treatment and low (0–8.3%) in HBsAg-negative/anti-HBc-positive patients. The ACR 2021 guideline strongly recommends that TNFi should be avoided in patients with untreated chronic HBV infection or with chronic HBV infection with significant liver injury (Child-Pugh class B or C). Moreover, the guideline recommends prophylactic use of antiviral drugs over frequent monitoring alone for patients with positive HBsAg. Frequent monitoring of viral load and liver enzymes is conditionally recommended over prophylactic antiviral therapy for patients who are anti-HBc-positive and HBsAg-negative and all patients should be co-managed with a hepatologist [11]. Therefore, TNFi should be avoided in patients with untreated hepatitis B, but it may be safe in patients receiving treatment of hepatitis B. For people with positive HBsAg, antiviral therapy (e.g., tenofovir or entecavir) should be started one week prior to starting TNFi. Antiviral therapy should be continued for at least 6 months after stopping TNFi. For HBsAg-negative but HBcAb-positive (resolved HBV infection) patients, monitoring for viral reactivation during TNFi therapy is recommended by checking HBV DNA and transaminase levels before starting therapy and every 3 months thereafter. Some experts monitor HBV DNA and transaminases at more frequent intervals (i.e., every 1 to 2 months) [14].
Although there are reports that treating HCV-infected patients with TNFi does not lead to further liver damage, even without concomitant antiviral therapy, guidelines recommend screening for anti-HCV antibodies in all patients [24]. A study on 20 RA patients with positive anti-HCV antibodies and HCV RNA after 1 year of TNFi therapy showed similar outcomes in RA patients with and without concomitant HCV infection. Also, in a study on 104 patients with positive HCV, no significant reactivation were seen after starting TNFi [25]. There was a report of 2 cases of hepatocellular carcinoma after long-term use of etanercept in HCV-positive patients with psoriasis and cirrhosis [16]. Tumor necrosis factor inhibitors are safe in untreated hepatitis C, although frequent liver enzyme monitoring is advised. All patients with confirmed hepatitis C infection should be referred to a hepatologist/gastroenterologist for consideration of treatment [8, 26].
Rituximab
No dose adjustment was suggested in the manufacturer’s labeling. It was used in multiple studies in patients with cirrhosis due to autoimmune hepatitis (AIH) or primary biliary cirrhosis with or without rheumatic diseases [27]. In a study on 35 patients with serious AIH (10 patients with cirrhosis), it caused a significant reduction in GC dose and the discontinuation of ≥ 1 immuno-suppressant in 47% of patients without serious side effects [28].
The risk of HBVr is very high (10–20%) with RTX [14, 29]. The ACR strongly recommends prophylactic antiviral therapy and frequent monitoring of viral load and liver enzymes for patients receiving RTX who are anti-HBc-positive (regardless of HBsAg status) [11]. For patients at risk of HBVr, antiviral therapy should be initiated 1 week before starting treatment with RTX and should continue for at least 12 months after stopping RTX treatment. Patients with rheumatic disease and cancer need prolonged, possibly life-long antiviral therapy [14, 29]. In a study, following 8 patients with diffuse large B cell lymphoma and hepatitis B-related cirrhosis treated with RTX-containing chemotherapy who were receiving standardized antiviral therapy, HBVr with positive HBsAg and detectable HBV DNA was observed in only one patient who was HBsAg negative before treatment, with no other hepatitis flares or abnormal liver function occurrence [29].
In patients with chronic hepatitis C, RTX is not expected to increase viremia. There could be a role for RTX depleting CD19 positive-B cells, known to be HCV reservoirs. Patients with HCV-related cryoglobulinemic vasculitis, especially those presenting with glomerulonephritis and/or neuropathy, should be treated with an RTX-based protocol. Antiviral therapy may be started at the same time as RTX therapy or may be delayed [30, 31]. On the other hand, RTX, by causing a decline in exosomal microRNAs, could theoretically be a risk for increased viremia in hepatitis C.
Therefore, treatment with RTX in patients with hepatitis C is recommended to be started under the supervision of a hepatologist [26]. There is no report of fulminant hepatitis C after using RTX. However, there have been rare case reports of HCV reactivation after RTX combination chemotherapy or in combination with high-dose GCs [32–34].
Tocilizumab
This anti-interleukin-6 monoclonal antibody should not be used if baseline AST or ALT > 1.5 ×, according to the recommendation of the manufacturer.
Tocilizumab (TCZ) therapy has been reported to be associated with HBVr in patients with chronic hepatitis B, even resulting in liver failure and a need for liver transplantation [35]. The risk of HBVr has been reported as “moderate” or “intermediate” (1–10% chance) [36]. Multiple recent studies based on the treatment of COVID-19 infection have reported that the risk of HBVr ranged from 0 to 60% in HBsAg-positive patients who underwent TCZ therapy without antiviral prophylaxis [37]. Moreover, in a study on patients with RA, the risk was very high (3/3; 100%) for HBsAg-positive patients who were not receiving prophylactic antiviral treatment, especially within the first year of treatment with TCZ and lower (1/64, 1.6%) among HBsAg-negative/HBcAb-positive patients [37]. In 2 studies also no HBVr occurred after TCZ treatment in 12 patients with previously resolved HBV infection [13]. Therefore, frequent monitoring of viral load and liver enzymes is recommended over prophylactic antiviral therapy for HBcAb-positive and HBsAg-negative patients who have started treatment with TCZ [11]. The HBsAg-positive patients require prophylactic antiviral treatment. It has been reported that HBsAg-negative/anti-HBc-positive patients with RA without anti-HBs antibodies had a significantly higher risk of HBVr after treatment with TCZ therapy; also, the presence of anti-HBs may have a protective effect in those patients [38].
In HCV-infected patients with RA, viral load is reported to be unaffected by short-term or 1-year treatment with TCZ [26]. Also, there was a case report of a patient with chronic hepatitis C virus, treated with long-term TCZ for her refractory adult-onset Still’s disease, who had good hepatic tolerance [39]. Again, collaboration between rheumatologists and hepatologists for treatment is recommended.
Janus kinase inhibitors
It is recommended by the manufacturer that tofacitinib (TOF) should be avoided in patients with severe hepatic impairment. Dosage adjustment is recommended in patients with moderate hepatic impairment. No dosage adjustment is needed in patients with mild liver disease.
Tofacitinib has 70% hepatic metabolism and 30% renal excretion. Thus, its dose should be adjusted in patients with Child-Pugh class B cirrhosis and it should be avoided in class C [8]. In moderate liver impairment, it is recommended that the dose should be reduced to half, and the dose frequency should be reduced to once daily in cases of using extended-release tablets or it should be changed to immediate-release tablets. Tofacitinib should be avoided in severe liver impairment in patients with chronic hepatitis B and C.
Baricitinib is primarily eliminated by renal excretion; therefore, according to the manufacturer it is recommended to be avoided only in cases of severe liver impairment. It can be used with no dose adjustment in mild to moderate liver impairment [10].
Upadacitinib has been recommended to be avoided in severe hepatic impairment. It has been shown that mild or moderate hepatic impairment has no clinically relevant effect on upadacitinib metabolism; thus, dose adjustment is not needed in RA patients with mild or moderate hepatic impairment [40].
Janus kinase inhibitors (JAKi) could induce HBVr in both HBsAg-positive and HBsAg-negative/HBcAb-positive RA patients. In HBsAg-positive patients, the risk of HBVr is high and antiviral prophylaxis is recommended. The risk of reactivation is low in HBsAg-negative/HBcAb-positive patients, and frequent monitoring of viral load and liver enzymes is recommended over prophylactic antiviral therapy [11, 41–43]. In a retrospective study, of 6 HBsAg-positive patients using TOF, 2 out of 4 developed HBVr, with no HBVr in 2 patients receiving prophylaxis. None of the 75 HBsAg negative/HBcAb-positive patients demonstrated HBVr. For baricitinib, 4 out of 215 patients with HBcAb-positive RA developed HBVr [13].
Janus kinase inhibitors have not been shown to affect HCV replication in patients with RA [26]. There was a report of severe hepatitis E in a patient receiving baricitinib with improvement after discontinuation of the medication [44]. A retrospective study and review that compared 4 JAK inhibitors concluded that baricitinib had the highest risk and TOF and upadacitinib had the lowest risk of hepatitis B reactivation [45].
Abatacept
According to the manufacturer’s recommendation, there is no need for dose adjustment in patients with liver disease, and no formal studies have been conducted to assess the effects of renal or hepatic impairment on its pharmacokinetics. Abatacept (ABT) has a medium risk (1–10%) for HBVr in HBsAg-positive patients and a low risk (< 1%) in HBsAg-negative and anti-HBC-positive RA patients [24]. In a retrospective study of international pharmacovigilance databases searching for all cases of HBVr between January 1, 2006 and June 30, 2021, there were 55 cases of HBVr with ABT, so they reported a positive association between ABT exposure and HBVr, and using antiviral prophylaxis when using ABT in patients with rheumatic diseases was recommended [2, 46]. Also, based on the American Gastroenterological Association (AGA) Institute recommendation, antiviral therapy should be considered, especially in patients with comorbidities, such as HBsAb-negative, using GCs, old age, and using other immunosuppressive agents [47]. Based on APASL clinical practice guidelines on hepatitis B reactivation related to the use of immunosuppressive therapy, in HBsAg-negative and anti-HBC-positive RA patients, no prophylaxis is recommended until further evidence is found [2].
It may suppress HCV viral replication and significantly decrease HCV viral load in patients with chronic hepatitis C. It was found that HCV RNA might become undetectable after costimulatory blockade, so it can be used in HCV-positive patients with the collaboration of a hepatologist [26]. In a study on 27 HBsAg-negative and anti-HBC-positive RA patients, 19% of those who used anti-viral prophylaxis did not have any HBVr [13].
Prednisone
Prednisone needs to be converted to the active form of prednisolone by the liver; therefore, in patients with hepatic function impairment prednisolone or methylprednisolone is preferred [8]. Patients with low levels of albumin resulting from liver diseases are more susceptible to the effects, including adverse effects, of GCs, so dosage adjustment should be considered. Moreover, in patients with cirrhosis, the clearance of unbound GCs is reduced to two-thirds compared with patients without liver disease [10].
Depending on the dose and duration of treatment, administration of prednisolone or methylprednisolone alone or in combination with other immunosuppressive agents can increase the risk of HBVr. The AGA guidelines divided the patients into 3 categories: high risk (dose of > 10 mg and treatment duration of > 4 weeks); medium risk (dose of < 10 mg and treatment duration of > 4 weeks); and low risk (dose of < 10 mg [2] and treatment duration of < 1 week) based on the dose and duration of treatment. Based on viral serology, HBsAg-negative/anti-HBc-positive patients are considered low risk (< 1%); thus, HBV DNA and ALT should be regularly monitored during therapy [14].
There are concerns about the risk of increased HCV viral replication with GCs. In most of the studies that reported an increased risk of viral reactivation, GCs were administered in combination with other immunosuppressive or chemotherapy agents. However, there are some case reports on increased HCV viral replication with GC monotherapy [48, 49]. Hepatology consultation is of value in patients with chronic hepatitis C infection requiring treatment with GCs.
Acetaminophen
According to the manufacturer’s instructions, it is contraindicated in patients with severe hepatic impairment or severe active liver disease and should be used with caution in patients with hepatic impairment or active liver disease. However, it can be safely used when prescribed in lower doses with maximum daily dose of 2 g for short durations in patients with cirrhosis [20].
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided in patients with advanced cirrhosis [20]. It is recommended that the dose should be reduced to 50% in severe hepatobiliary diseases. The increased risk of gastrointestinal bleeding should be considered in these patients [8].
Non-steroidal anti-inflammatory drugs are associated with an increased risk of nephrotoxicity, reduced glomerular filtration rate, and impaired renal perfusion in patients with cirrhosis and should be avoided in patients with cirrhosis [50].
Due to the high risk of liver toxicity, diclofenac and sulindac should be avoided in liver diseases [10]. Moreover, indomethacin should be avoided in patients with impaired biliary function since it has biliary excretion and undergoes enterohepatic circulation [8].
Narcotics
Narcotics (opioids) are mostly metabolized by the liver. Morphine has a high oral bioavailability and a long elimination half-life. The metabolism of morphine is impaired significantly in patients with liver cirrhosis, so it should be used with caution [51]. Fentanyl and hydromorphone are the safest narcotics, but the modifications for lowering the dose or increasing the dose interval should be considered in patients with liver cirrhosis [52]. Meperidine should be avoided in patients with liver disease [8]. Narcotic use is associated with increased risk of hepatic encephalopathy in patients with cirrhosis and advanced liver disease and should be avoided [53].
Tramadol
According to the manufacturer’s labeling, adjustment of the dose of tramadol is recommended in patients with liver disease and cirrhosis. The dosing interval of tramadol should be increased from 6 to 12 hours. Tramadol should be started with a lower dose of 25 mg in patients with liver disease, and the dose should not be greater than 50 mg every 12 hours. The extended-release formulations should be avoided [8]. The possible effect of tramadol on slowing of bowel movements and increased risk of hepatic encephalopathy should be considered in patients with significant liver disease.
Medications used in systemic lupus erythematosus
Immunosuppressants in patients at high risk of HBVr should be started after 1 week of receiving antiviral therapy, and antiviral therapy should be continued for at least 6 months after the cessation of immunosuppressive therapy [14]. Increased viral replication may occur in HCV-infected patients [28].
Azathioprine
It has been recommended by its manufacturer that it should be used with caution in severe liver disease. The effects, including adverse effects, may be increased due to slower metabolism of medication. Azathioprine can cause increased viral replication of hepatitis virus B and C [54]. Due to the lack of data of its harmfulness and based on the APASL (Asian Pacific Association for the Study of the Liver) clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy, the risk of HBVr in HBsAg-positive and HBsAg-negative/anti-HBc-positive patients is low (< 1%) [2, 13].
Cyclophosphamide
Cyclophosphamide (CP) is rapidly metabolized, mainly by the liver, into active and inactive metabolites. Therefore, in patients with advanced liver disease, the conversion to the active form may be compromised, leading to potentially reduced efficacy. Cyclophosphamide can be used in mild to moderate liver disease. However, it should be avoided in severe liver disease due to reduced efficacy. The half-life of CP is about 8 hours and is increased to 12 hours in patients with hepatic impairment. Nevertheless, CP level is not increased in patients with liver disease, which suggests that dose modification is not required in such patients [10]. As CP is commonly used along with GCs in the treatment of patients with rheumatic diseases, it is difficult to find the exact risk of HBVr based on limited data. In a study on 138 rheumatic disease patients, HBVr was seen in 11 (8%) after a median time of 8 weeks of CP treatment in 99 (72%) HBsAg-positive and 39 (28%) HBsAg-negative/HBcAb-positive patients [13]. Thus, it should be considered as high-risk for HBVr in HBsAg-positive patients, but low risk in HBsAg-negative and anti-HBC-positive patients [13].
Calcineurin inhibitors – cyclosporine and tacrolimus
In patients with liver disease, the excretion of cyclosporine (CsA) and tacrolimus (TAC) metabolites is impaired, and these agents should be avoided in severe liver disease [10]. According to the manufacturer’s instructions, as they are extensively metabolized by the liver, severe hepatic impairment may result in a significantly increased level of medication. In these conditions, the dose of medications should be reduced, and their blood levels should be monitored frequently. Cyclosporine is excreted in the bile and undergoes enterohepatic circulation. It should be avoided in patients with impaired biliary excretion [8].
The metabolism of TAC is dependent on the expression and activity of hepatic CYP3A4 and CYP3A5 enzymes, and about 95% of its metabolites are excreted via the biliary route. This may lead to a higher blood level of TAC in patients with hepatic dysfunction [55].
Cyclosporine has a moderate (1–10%) risk of HBVr. HBsAg-positive patients starting this medication should be considered candidates for prophylactic anti-HBV therapy [16]. Cyclosporine in anti-HBc-positive/HBsAg-negative patients with RA has not been associated with increased risk of HBVr, so only close monitoring during treatment is recommended [16]. Cyclosporine, but not TAC, suppresses the replication of HCV both in vitro and in vivo, in a dose-dependent manner [56, 57]. The safety of CsA in HCV-positive RA patients has been evaluated in combination with TNFi, and no significant adverse event has been observed [58]. In a study on 11 psoriasis patients with concomitant HCV infection who used CsA for 16–38 months, no exacerbation of HCV was detected [16].
Mycophenolate mofetil
There is no need to change the dose of mycophenolate mofetil in patients with liver disease, but doses more than 2 g/day are advised to be avoided. It can increase HCV and HBV replication due to its immunosuppressive mechanism [54]. However, according to a previous retrospective study on patients with systemic lupus erythematosus and chronic or resolved HBV infection from Taiwan, it did not show HBVr, so it was considered a low-risk drug for HBVr [13].
Belimumab
Changes in hepatic function are unlikely to have any effect on its elimination, according to the manufacturer. Physicians should be cautious in patients with chronic infections. Although it was not recommended to check for a hepatitis panel before starting this medication, reactivation of hepatitis B leading to fatal fulminant hepatic failure has been reported in one case [59]. Taking into consideration currently insufficient research on the risk of HBVr, prophylaxis for HBVr can potentially be recommended [13].
Dapsone
There is no need for dose adjustment in patients with liver disease; however, it should be used with caution [60].
Medications used in spondyloarthropathies
Secukinumab
It has been observed that in the absence of antiviral prophylaxis, up to 15.2% of patients with HBV exhibited viral reactivation, especially in HBsAg-positive patients. In HBsAg-negative/HBcAb-positive patients receiving antiviral prophylaxis, the risk of reactivation is very low. The incidence of HCV reactivation is reported to be very low [61]; however, the patient should be monitored and treated with the cooperation of a hepatologist, as recommended for TNFi [16]. In a retrospective study on 37 patients with axial spondyloarthritis and concurrent HBV infection, 6 (16.2%) patients developed HBVr after using secukinumab for 9.0 ±5.7 months, whether they had received antiviral prophylaxis or not [62]. Another multicenter study on 46 HBsAg-positive psoriatic patients on secukinumab without receiving antiviral prophylaxis reported 7 (15.2%) patients with HBVr although other studies using concomitant antiviral prophylaxis had a very low risk of viral replication [63]. Thus, it was recommended that prophylaxis antiviral should be suggested on a case-by-case basis in HBsAg-negative/HBcAb-positive patients. The incidence of HCV reactivation is very low but it is still possible in patients with chronic HCV positivity [63]. There was only one reactivation in 18 patients with HCV infection, and considering TNFi has been recommended until now [16].
Ixekizumab
The precautions needed during the use of ixekizumab in patients with liver disease are similar to those for TNFi in this group of patients [16].
Apremilast
Apremilast is not contraindicated in patients with liver disease including those with severe liver function impairment [64]. No significant hepatotoxic effect is expected, and there is no need for dose adjustment in moderate or even severe hepatic impairment (Child-Pugh B and C) based on its manufacturer’s instructions. This agent is also possibly safe in HCV- and HBV-infected patients [16]. It does not require pretest screening for hepatitis B and C [63]. Patients with hepatitis B infection (HBsAg-positive) patients have a low risk of viral replication. Hepatitis B surface antigen-negative/anti-HBc-positive patients also have a low risk of reactivation and do not require anti-HBV therapy, nor should monitoring be considered mandatory [16].
Ustekinumab
Patients with hepatitis B infection (HBsAg-positive) carry a high risk of HBVr and should be considered for prophylactic antiviral therapy [16]. Hepatitis B surface antigen-negative/anti-HBc-positive patients have a moderate risk of reactivation (1–10%) and should be monitored closely during treatment [16]. In a study carried out on 11 HCV-positive patients with psoriasis including 3 with concomitant HBV infection, taking ustekinumab, there was no evidence of viral reactivation or significant elevations in liver enzymes [65]. However, in HBsAg-positive patients with psoriasis, 2 studies showed 28.6% and 25% HBVr with no antiviral prophylaxis usage [13]. One reactivation out of a total of 9 patients in one study and one reactivation and 2 cases of a slight rise in HCV RNA in 4 patients have been reported in a few available studies [16].
Medications used in gout
Allopurinol
Allopurinol is not contraindicated in patients with cirrhosis. There is no need for dose adjustment in patients with liver disease or cirrhosis. However, due to rare cases of drug-induced liver injury, the manufacturer recommended that it should be used with caution in patients with liver disease [8, 66].
Febuxostat
Febuxostat is metabolized by the liver and should not be used in patients with moderate to severe liver disease [10]. Febuxostat 80 mg once daily was found to be safe and well tolerated in patients with mildly to moderately impaired hepatic function, and dose adjustment was not required [67].
Colchicine
The manufacturer recommended that adjustment of the dose is not required in patients with mild to moderate hepatic impairment, but patients should be monitored closely for adverse effects. The clearance of colchicine may be significantly reduced with prolonged plasma half-life in patients with chronic hepatic impairment, compared to healthy subjects [68]. The main route of elimination of colchicine is hepatobiliary excretion, so the risk of toxicity is increased in patients with cirrhosis, and in moderate liver dysfunction dose reduction is recommended [69].
Medications used in osteoporosis
Bisphosphonates
There is no need for dose adjustment in patients with liver disease [70]. In a study, 57 patients with liver cirrhosis and esophageal varices received oral risedronate at 35 mg weekly plus daily calcium and vitamin D supplementation for the treatment of osteoporosis. The improvement in T score with low bleeding risk under endoscopic surveillance was not significantly different from the control group [71].
Denosumab and parathormone
There is no need for dosage adjustment in patients with liver disease, as described in the manufacturer’s recommendations. Denosumab treatment was found to be safe and effective in 60 patients with chronic liver disease diagnosed with osteoporosis [72]. Teriparatide has not been studied in patients with liver disease.
Conclusions
Treatment of patients with rheumatic disease and severe hepatobiliary disease may be challenging. In addition, there are concerns about viral reactivation during treatment with immunosuppressants and biologic medications in patients with chronic viral hepatitis. Therefore, many precautions should be considered; in most cases, collaboration with a hepatologist is required.