Introduction
Ankylosing spondylitis (AS) is a chronic, inflammatory, progressive rheumatic disease. Axial AS occurs with the involvement of the sacroiliac joints, spine, ligaments and peri-spinal tissues. Diagnosis of AS can be established when the modified New York Criteria are met [1].
In the course of severe disease the mobility of the spine and chest decreases until they become completely rigid [1, 2]. Other symptoms are pain and morning spine stiffness, which subsides with movement. At the onset of the disease, the pain is usually reported within the sacroiliac joints and the upper lumbar and lower thoracic spine [3].
As the disease progresses, the patient’s body shape changes, which results from the vertical position of the sacrum, the loss of lumbar lordosis, excessive thoracic kyphosis and cervical lordosis. The head and shoulders protrude, and the shoulder blades move away from the spine. The cooperation of the muscular system is disturbed [4].
Improvement in AS depends on the activity and the stage of the disease. Regular, properly selected physiotherapy and physical activity increase the patient’s efficiency and delay the deformation of the patient’s body. Lack of movement and staying in a sitting position for a long time promote spinal deformity and strengthen peripheral joint contractures [5].
As the disease progresses, the patient’s physical capacity decreases, which results from abnormal chest movement patterns. Progression limiting the mobility of the chest affects the mechanics of breathing and leads to malfunction of the lungs and bronchi [6, 7].
Rehabilitation is a very important element of treatment, thanks to which the patient has a chance to maintain functional efficiency at the highest possible level. Therefore, new methods of improving and monitoring treatment progress are constantly being sought.
The aim of the study was to assess the usefulness of the 4D body technology (4DBODY) to assess the effectiveness of AS treatment.
Material and methods
Study design
To evaluate the 4DBODY technology a patient with confirmed AS was chosen [8].
To use the 4DBODY system the medical data of the studied patient were analyzed. The patient complained of chronic back pain, especially in the lumbar and the thoraco-lumbar regions and presented symptoms of inflammation of the spine junction and right Achilles tendon.
Additionally at the age of 12 the patient underwent a corrective surgery due to juvenile avascular necrosis of the left femur and finally in 2019 underwent a total left hip joint replacement.
According to therapeutic recommendations for AS the patient was treated with non-steroidal anti-inflammatory drugs (NSAIDs). Also the patient each year underwent a 3-weeks rehabilitation program in the department of rehabilitation. For the last 2 years the patient had the same therapeutic regimen before rehabilitation in which the 4DBODY system was used.
Device type
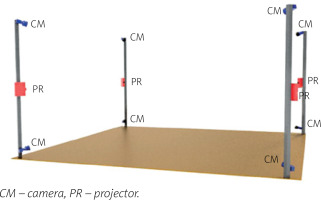
The 4DBODY system consists of 8 detectors and 4 projectors divided into 4 measuring heads, which are evenly distributed around the center of the measuring volume (Fig. 1) [9].
The system uses FLIR Grasshopper 3.0 detectors with a 2.3-megapixel sensor providing the acquisition frequency of up to 163 Hz (reduced to 120 Hz when capturing is synchronized with projectors). The projectors are Casio XJ-A242 with the resolution of 1,280 x 800 pixels and a hybrid light source of 2,500 lumens that combines laser and LED technology.
This system utilizes a structured light projection technique, in a single frame variant based on a sinusoidal fringe pattern. Neighboring measuring heads are spectrally separated, which makes it possible to capture the entire surface of the human body in motion. The results are in the form of point clouds reflecting the actual measurement coordinates. The 4DBODY system ensures that the spatial resolution of the output point cloud is not greater than 1.0 mm and its measurement uncertainty is not greater than 0.5 mm.
Measurement procedure
The standing patient was positioned within the measurement volume of the scanning system. The complete breathing cycle was measured, so the patient was asked to take a deep breath and exhale. Chest movement was recorded while breathing. A sequence of point clouds was recorded. Then MATLAB R2019b software was used to analyze whether the applied therapy had any influence on chest movement.
At first, a few consecutive operations consisting of experimentally selected translations and rotations were applied to the data to position the shoulders line parallely to the Y axis (left – right axis) in the XY plane (axial plane). Such orientation of the data, resembling the LPS (left, posterior, superior) orientation from the anatomical coordinate system, simplified further processing.
The majority of points were removed from about 1.8 million points present in the initial cloud in order to minimize the duration of calculations. A 100-mm high fragment bounded by two planes parallel to the XY plane was left.
The lower separating plane was placed slightly higher than the connective element of the bra, while the upper one was slightly lower than the shoulder line. The resulting cloud included approximately 150,000 points. Next outliers were removed from this set [10] to eliminate the measurement noise understood as sparse outliers.
The neighborhood parameter for this action was set at 100 points, and the cut-off point at 2.5 mm. To reduce the effect of swaying in the frontal plane, i.e. moving sideways, the point of gravity of the cloud was calculated on its 10 mm high fragment, and the cloud was repositioned to a assumed common position of all clouds in the sequence.
The next step involved a selection of a plane parallel to the XY plane. The lower plane whose neighborhood, for the whole sequence, was not obscured by the bra was selected. Points for analysis were taken from that area. The neighborhood was defined at ±0.5 mm.
The points were projected onto the XY plane and further analyses were conducted in the 2D space. Subsequently, the points were sorted on the X axis which facilitated their division into the anterior and posterior section. Each of those sections was processed independently in the same manner.
First, the points were sorted on the Y axis to be filtered with the median filter with a 15-point wide window. Next, cubic spline data interpolation was used to determine points distributed evenly every 0.2 mm on the Y axis. It was then possible to calculate the distance between the front and back segment for each of the above-mentioned points on the Y axis.
We analyzed the movement of the point located on the body of the sternum against the point which was its projection on the X axis on the part of the cloud representing the patient’s back. The position of the above mentioned points was determined for each cloud of the measurement sequence.
Results
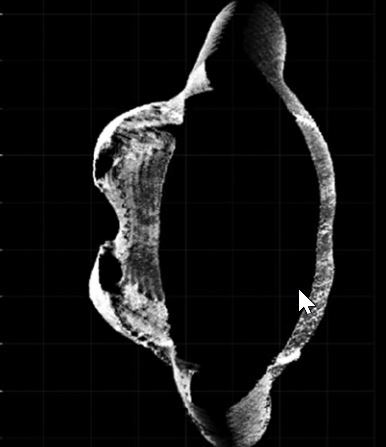
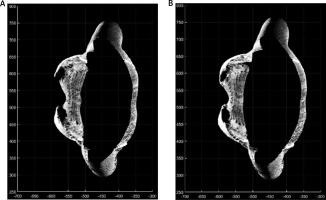
Positive changes were observed in the assessment of chest activity with the 4DBODY system. Figure 2 shows two fragments of point clouds, each 100 mm in height, visualized on the XY plane. The shape of the chest visibly changed during breathing. As mentioned before, two points (interior site of the chest in points mostly prominent in the axial plane) on the patient’s body were observed (Fig. 2).
Fig. 2
The XY-plane view of the fragments of point clouds reflecting the shape of the thoracic segment of the trunk on expiration (A) and inspiration (B).
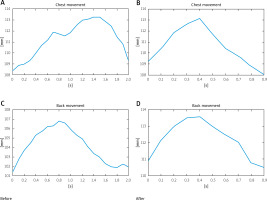
Their relative movement is presented in Figure 3. Notably, prior to the rehabilitation the movement of the sternum was phased out in relation to the movement of the back. On inhalation, a movement in the back was observable (leaning back). It seems as if the patient used this movement to compensate for the problems with the mobility of the sternum.
Afterwards, the movement of the sternum was visible. The reflex regressed after the rehabilitation, and both the movement of the sternum and the back started and finished at the same moment. The amplitude of chest swing also changed.
Prior to the rehabilitation the range of motion between those two measurement points was 18 mm, while after the rehabilitation it increased almost by 10 mm to 27.9 mm (Table I).
Table I
Minimal and maximal distance between two selected measurement points recorded on expiration (Min) and inspiration (Max) and the difference between them (Range) before and after therapy
| Before therapy | After therapy | ||
|---|---|---|---|
| Min [mm] | Max [mm] | Min [mm] | Max [mm] |
| 200.5 | 218.5 | 198.8 | 227.7 |
| Range [mm] | Range [mm] | ||
| 18.0 | 27.9 | ||
We also observed changes in the angular values of spinal orientation. As regards the lumbosacral segment the lordosis was reduced from 33° to 22°. The smallest change occurred in the thoracic spine – only 2° of kyphosis reduction. In the cervical spine the kyphosis was reduced from 44° to 39°.
Chest expansion was also improved from 25 mm prior to the treatment to 50 mm after the treatment measured at the level of the fourth intercostal space, and from 30 mm to 50 mm at Th10 level (Table II).
Table II
Physiotherapeutic evaluation before and after therapy
The present study showed that the traditional measurement revealed the expansion of the chest of 25 mm, while computed measurement revealed 18 mm in the sagittal plane prior to the therapy at the level of the fourth intercostal space. After the therapy the expansion of the chest was 50 mm based on traditional measurement and 28 mm in the sagittal plane at the level of the fourth intercostal space.
Notably, the classic method of measurement refers to the circumference of the chest, while computed quantification only shows the sagittal dimension. Therefore, the therapy was effective not only in the sagittal dimension of the chest, but also in the complete three-plane mechanics of the ribs and chest.
Plethysmography revealed a significant increase in the respiratory muscle strength in the patient. Inspiratory muscle strength (MIP – maximal inspiratory pressure) increased from 80% to 93% of the reference ranges for the patient. Expiratory muscle strength (MEP – maximal expiratory pressure) increased from 46% to 86%. The remaining plethysmographic parameters were not so significantly changed (Table III).
Discussion
Ankylosing spondylitis patients require constant monitoring in terms of changes within the musculoskeletal system and the regular rehabilitation of the chest. Numerous methods are used for the visualization of chest mobility of AS patients.
The respiratory movement measuring instrument (RMMI) is one of the most common instruments used to visualize chest mobility. The method involves the calculation of chest mobility during inhalation using laser markers.
The patient is in a supine position, which is a disadvantage of this method, as the posterior part of the chest is excluded from the respiratory movement and the patient breathes under anti-gravitational conditions [11–13].
Optoelectronic plethysmography is another method of visualizing chest mobility. However, attaching a considerable number of markers is time-consuming, and the respiratory movement of the patient affects the sensitivity of the examination [14–19].
Moreover, a magnetometer [20] may also be used, but it mostly measures cranio-sacral movement and needs to be calibrated with a spirometer and a pneumotachograph every time. Therefore, it is very difficult to use and it is associated with a considerable number of errors [21].
Three-dimensional X-ray computed tomography (CT) using the dynamic spatial reconstructor is another form of chest imaging, but the calculation processes are performed too slowly [17].
As regards ELITE and other systems based on the analysis of markers attached to the patient’s body, it was difficult to attach the markers precisely on the patient’s body [21–24]. Furthermore, there were attempts at using imaging tests such as ultrasound [25] or CT [26] in the visualization of chest movement. It was difficult, though, due to the fact that they are primarily used for other purposes.
Ultrasound examination is additionally limited by the skills of the diagnostician and the calibration of the device in every case. Moreover, it only visualizes individual structures participating in respiration, e.g. the diaphragm, but it does not include the complete respiratory cycle.
Computed tomography is much more effective in the diagnosis of structural primary or secondary intrapulmonary lesions, but assessment of the respiratory cycle is problematic. Additionally, the patient is exposed to unfavorable radiation.
Moreover, during ultrasound or CT examinations the patient is in a lying position, with the back or side to the measurement devices, which affects the mechanics of the chest. The 4D imaging was developed thanks to the most recent technological achievements. Its effectiveness was confirmed in the present study and it may be recommended for the assessment of chest activity and monitoring therapeutic outcomes in AS patients.
The rigidity of the chest and its joints combined with pain and an abnormal respiratory pattern may lead to decreasing pulmonary function [27] and weakening respiratory muscles [28]. Research conducted by Sahin et al. [29] revealed that AS patients were characterized by significantly lower chest expansion, respiratory muscle weakness, FVC and FEV1 parameters.
Reddy et al. [30] found a significant correlation between chest expansion and the following parameters: forced vital capacity (FVC), forced expiratory volume in one second (FEV1), vital capacity (VC), and FVC/FEV. Numerous authors have reported a strong correlation between the degree of chest expansion and pulmonary function and the degree of degradation of the costotransverse and costovertebral joints [31].
Apparently, even the presence of grade 4 radiological lesions according to the New York sacroiliitis radiological grading criteria [33] does not rule out the possibility of improving chest mobility in AS patients. Chest mobility is a very important quantification parameter in this group of patients. Research revealed a significant correlation between chest mobility, respiratory muscle strength and appropriate pulmonary function [32, 33].
Chest mobility is very important in terms of the functional disorders of pulmonary function and structural changes as well. Apical pulmonary fibrosis is the most common structural lesion within the pulmonary tissue in patients with AS.
The pathology occurs as a result of chest rigidity, particularly in the upper region, and in the area of the cervicothoracic junction and the scapulae. It results in reduced functional pulmonary volume and obstructed inflation of this area [34].
Therefore, it is necessary to work on the chest in AS patients, which is confirmed by the improvement obtained in most of the measurements carried out in our patient. Breathing exercises are an effective and necessary element of rehabilitation, and the use of the system can be used to very precisely assess chest dysfunction and monitor its improvement in its function.
Study limitations
This study focused on the method and analysis based on data of one patient with a well-established AS diagnosis. However, the authors of this article are aware that the introduction of the system to the standard assessment of rehabilitation of AS patients requires further research in larger groups of patients.
Conclusions
Thanks to the use of the 4D scanning system, it was possible to demonstrate a multifactorial, reliable and maximally objective improvement in respiratory parameters.
The results obtained with the 4DBODY system correspond to the results of the physiotherapeutic assessment, which confirms the value of its use in treatment monitoring. From our point of view, the 4DBODY system is a promising tool for visualizing the progress of chest rehabilitation in patients with AS.