Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease causing chronic inflammation. The pathogenesis of RA involves a complex network of various cells and cytokines. Involvement of the tumor necrosis factor α (TNF-α) is central to the pathogenesis of RA [1–3]. Biologic treatment with TNF-α inhibitors has definitively proven a critical role for TNF-α [4]. However, the pathogenesis of RA remains incompletely understood, and new immune pathways involved in the course of RA are still being sought [5, 6].
The importance of the kynurenine pathway in the normal function of the immune system has led to the appreciation of its possible contribution to autoimmune diseases such as RA. Elevated levels of pro-inflammatory cytokines (such as TNF) are probably able to activate indoleamine 2,3-dioxygenase (IDO) – an enzyme catabolizing tryptophan to kynurenine. On the other hand, long-term regulation of autoimmune diseases may be dependent on epigenetic modulation of kynurenine pathway genes. The IDO/kynurenine pathway is now established as a regulator of inflammation processes [7–9]. It is also possible that the IDO/kynurenine pathway induces neurological symptoms and contributes to the discrepancy between symptoms and signs, including symptoms of hyperalgesia and depression in patients with rheumatoid arthritis [10]. However, the role of the kynurenine pathway in rheumatoid arthritis is not clear.
Most of the studies showed that, compared with healthy controls, RA patients had a higher kynurenine-to-tryptophan ratio in serum, which suggests that increased IDO activity may be related to disease activity [11–14]. However, there is research demonstrating reduced concentrations of kynurenine in animal models of RA [15, 16]. Contradictory reports can also be found in studies on patients with RA [17–19]. The results are therefore not unequivocal. Even less is known about the impact of anti-cytokine treatment on IDO activity and the kynurenine pathway. To the best of our knowledge, only one such publication has been published. In 2011 Kurz et al. [20], in a study involving 22 patients, found that anti-TNF-α therapy does not influence IDO activity in patients with RA. However, more research is needed.
The aim of the present study was to evaluate the effect of therapy with TNF-α inhibitors on the activity of the kynurenine pathway in patients with RA. We believe that continuing elucidation of pathophysiological pathways relevant in RA offers substantial hope for the development of specific pharmacotherapy for treatment of RA – especially for comorbidity of RA and depression [10].
Material and methods
Patients
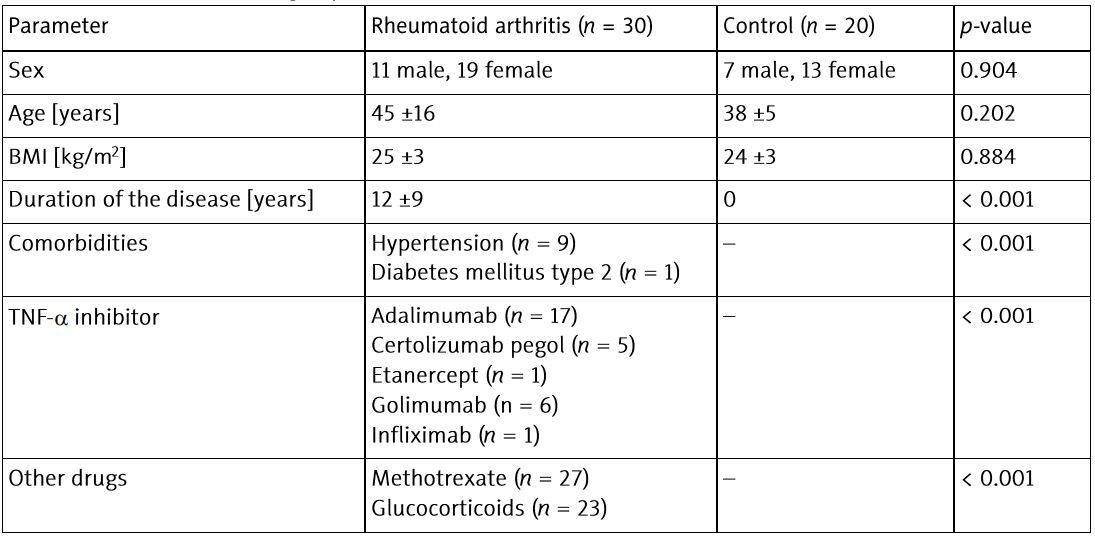
The study was performed on 30 patients with RA (Caucasian, 11 male, 19 female; mean age 45 ±16 years) treated with TNF-α inhibitors. All patients were assessed during selection for biological treatment and after 6 months of therapy.
The following inclusion criteria were adopted:
Exclusion criteria were:
heart, respiratory, kidney or liver failure,
acute or opportunistic infection in the last 3 months,
documented human immunodeficiency virus infection,
cancer (during the last 5 years),
age under 18 or over 65,
neurological and psychiatric diseases including depression,
pregnancy.
The patients were treated with TNF-α inhibitors (adalimumab, certolizumab, etanercept, golimumab or infliximab) as per standard protocols. According to European Alliance of Associations for Rheumatology (EULAR) guidelines [22], patients who received methotrexate (MTX) continued this therapy, while only 3 patients from the study group used anti-TNF-α agents in monotherapy – due to contraindications to MTX. There were no changes in the treatment of patients during the entire observation period. Patients receiving glucocorticosteroids (GCs) were included in the study only when the dose was below 5 mg of prednisone equivalent per day, at the same doses for at least 4 weeks before and during all the study. Patients who did not respond to TNF-α inhibitors were excluded from the analysis. Initially, 60 patients were included in the study. However, 30 of them were excluded from further analysis due to a change in treatment during the observation period (n = 18), lack of adequate response to therapy (n = 10) and loss to follow-up (n = 2).
As a control group, age- and sex-matched, 20 healthy volunteers were recruited. The participants in the control group (7 male and 13 female, mean age 38 ±5 years) did not have any chronic diseases or depression and were not taking any medications.
Clinical assessment of disease activity
Patients were examined before treatment and after 6 months of therapy with TNF-α inhibitors. Disease activity was assessed by the Modified Disease Activity Score (DAS) including tenderness and swelling in 28 joints, results of erythrocyte sedimentation rate (DAS28(ESR)) and Visual Analogue Scale (VAS) designated by the patients [23]. The response to therapy was defined according to the EULAR criteria [24]. If EULAR remission criteria were not met, the patients were considered to be non-responders and were excluded from further analysis.
Laboratory analysis
Blood samples were collected at the time of clinical examination – before treatment and after 6 months of therapy. In the control group, all measurements were made only once. Concentrations of kynurenine, serotonin and tryptophan in serum were determined with immunoassays (Immundiagnostik, Bensheim, Germany), with sensitivity of 1.46 μmol/l, 6.9 ng/ml and 0.12 μmol/l, for tryptophan, serotonin and kynurenine, respectively. The immunoassays were performed according to the manufacturer’s instructions. To estimate IDO activity, the kynurenine-to-tryptophan ratio was calculated. Other laboratory tests were performed using routine methods by the hospital laboratory.
Statistical analysis
The data were presented as medians and interquartile ranges or means and standard deviations, as appropriate. Since most of the data (except age and BMI) were not normally distributed (tested with the Shapiro-Wilk’s test), and due to the small number of patients in groups, all the data were analyzed with nonparametric tests. Unpaired data were analyzed with the χ2 or Mann-Whitney test, respectively. The Wilcoxon test was used to compare related parameters at the beginning and after treatment. Correlations between variables were analyzed with Spearman’s rank correlation coefficient. Statistical analyses were performed with the Statistica 13.0 software (StatSoft Polska, Krakow, Poland). A p-value < 0.05 was considered statistically significant.
Results
Characteristics of the study groups are presented in Table I.
Table I
Characteristics of the groups
Patients with RA were characterized by statistically significant differences in tryptophan metabolism pathways compared to the control group. As expected, RA patients had lower serum serotonin concentrations. However, RA patients also had a statistically significant lower kynurenine-to-tryptophan ratio – which could indicate lower IDO activation in RA (Table II).
Table II
Comparison of tryptophan metabolism in rheumatoid arthritis group (before treatment) and control group
Six months of TNF-α inhibitor treatment resulted in a significant improvement – 20 patients achieved remission (DAS28(ESR) < 2.6) and the remaining ten achieved low disease activity (DAS28(ESR) ≤ 3.2). The response to treatment was reflected by clinical improvement and laboratory criteria (Table III).
Table III
Clinical and biochemical parameters before and after 6 months of treatment with TNF-α inhibitors
In contrast, biological treatment only partially affected tryptophan metabolism, causing only an increase in serum kynurenine levels (Table IV).
Table IV
Tryptophan metabolism before and after 6 months of treatment with TNF-α inhibitors
In addition, concentration of tryptophan metabolites did not correlate with laboratory inflammatory markers or clinical disease activity.
Discussion
The results of our study demonstrated the altered tryptophan metabolism in patients with RA, compared with healthy controls. Interestingly, and to our mind also rather surprisingly, RA patients had a statistically significant decreased kynurenine-to-tryptophan ratio, which could indicate diminished IDO activation in RA. Additionally, RA patients had lower serum serotonin concentrations. The reason for this observation is unclear. In normal immune system function there is a balance between the serotonin and the kynurenine pathways. Only 5% of tryptophan is metabolized to serotonin and melatonin – although they play a very important role – while approximately 95% is converted to kynurenine or tryptophan, via IDO-1 and IDO-2, and 2,3-dioxygenase (TDO2), respectively. In healthy people, tryptophan is metabolized mainly by TDO2, while IDO plays a minor role [25]. This balance is disturbed by inflammation [26]. It has been demonstrated in vitro that TNF-α enhances IFN-γ induced tryptophan degradation by activation of the kynurenine pathway. In chronic inflammation, caused by rheumatic diseases, IDO-mediated tryptophan metabolism is activated, which at the same time reduces the production of serotonin [27]. Most of the studies reported in the literature showed that, compared with healthy controls, RA patients had a higher kynurenine-to-tryptophan ratio [11–14]. However, significant differences in the levels of kynurenine and kynurenine-to-tryptophan ratio (considered as an indicator of systemic IDO activity) between patients and control groups could not be found for all RA patients, suggesting the existence of disease heterogeneity based on the kynurenine pathway [17–19]. Moreover, in animal models for RA, it was found that in serum from diseased mice the concentration of kynurenine was reduced. These observations coincided with lower mRNA expression for IDO and increased mRNA expression for kynureninase in the liver during the disease. Therefore, decreased concentration of kynurenine in RA can be explained by two observations. Firstly, the tryptophan catabolism via the kynurenine pathway could be reduced in the liver during the disease. Secondly, catabolism might be increased in the liver. This could explain the decreased concentration of kynurenine in serum from diseased mice [15, 16]. However, it is uncertain whether a similar mechanism occurs in patients with RA. In the past, increased urinary excretion of kynurenine by patients with RA was also demonstrated [17, 28, 29]. The mechanisms affecting serum kynurenine levels in RA may therefore be more complex – as was detailed in a review [30]. Additionally, it should not be overlooked that although there is a close relationship between kynurenine metabolism and inflammatory responses, there are many other factors that may affect the kynurenine pathway, e.g. age, diet, microbiota, and physical activity [31, 32].
Moreover, we found no significant changes in kynurenine-to-tryptophan ratio (considered as an indicator of IDO activity) in RA patients treated with TNF-α inhibitors, despite clinical remission. Additionally, the kynurenine pathway activity did not correlate with disease activity or the laboratory markers of inflammation. However, we observed an increase in serum kynurenine levels in RA patients after successful treatment. There are different explanations for this observation. Overproduction of pro-inflammatory cytokines (such as TNF-α) contributes to altered tryptophan metabolism [33]. TNF-α is known for its ability to influence the kynurenine pathway. In human epithelial cells, interferon γ (IFN-γ)-induced IDO expression is transcriptionally enhanced by TNF-α [34]. Therefore, it can be expected that effective treatment of inflammation, by blocking TNF-α, could result in an improved balance in tryptophan metabolism. However, anti-TNF-α agents can exert an influence independently from IFN-γ. Probably TNF-α inhibitors could modulate the immune system without down-regulating biochemical pathways that are controlled by IFN-γ, such as the kynurenine pathway. But even in this case there should be an interaction between TNF-α and IFN-γ [35]. Another possible explanation for the fact that IDO activity did not change significantly after treatment is that TNF-α inhibitors represents a very effective symptomatic, but not causal therapy. Despite effective action, anti-TNF-α treatment does not modulate other, so far not elucidated pathways [36]. To the best of our knowledge, there is only one study available in the scientific literature examining the effect of TNF-α inhibitors on the balance between kynurenine and serotonin pathways in RA. Kurz et al. [20] stated that TNF-α inhibitors do not influence IDO activity in patients with RA, despite effective treatment of RA. No changes in the profiles of tryptophan metabolites were also reported in RA patients receiving prednisolone or MTX [11]. On the other hand, research in animal models suggested that inhibitors of cyclooxygenase-2 may have beneficial effects in major depression [37]. However, this requires confirmation in further studies.
An obvious limitation of our study is the small size of the cohort. An additional limitation is the lack of measurement of mRNA expression for IDO in the liver. Due to the limitations of our study, the results can only be regarded as preliminary. To investigate this observation, more research is needed.
Conclusions
The results of the study showed the altered tryptophan metabolism in RA patients, compared with healthy controls. Simultaneously, we found no significant changes in kynurenine-to-tryptophan ratio (considered as an indicator of IDO activity) in RA patients after treatment with TNF-α inhibitors, despite disease remission. Additionally, tryptophan metabolism did not correlate with disease activity or laboratory markers of inflammation, which may indicate that mechanisms affecting tryptophan metabolism and the role of the kynurenine pathway in RA may be more complex. We believe that understanding pathophysiological pathways relevant in RA offers substantial hope for the development of specific pharmacotherapy for treatment of RA – especially for comorbidity of RA and depression.