Introduction
Biological drug therapy appears to be a powerful tool for the treatment of chronic inflammatory rheumatic diseases and certain granulomatous pathologies that are resistant to conventional treatments. However, paradoxical effects have been reported in patients using various classes of medications, particularly the tumor necrosis factor α antagonists (anti-TNF-α) [1].
A paradoxical reaction is defined as the emergence of a condition that could be treated with biological therapy, the worsening of a pre-existing condition, or the development of a de novo paradoxical effect [2–4]. Therefore, the primary objective of our study was to assess the prevalence of paradoxical reactions in patients with inflammatory rheumatic diseases undergoing treatment with a biological drug. Additionally, we established secondary objectives to determine the types of paradoxical reactions and to investigate the associated factors.
Material and methods
The Biotherapies Registry of the Moroccan Society of Rheumatology registry
The Biotherapies Registry of the Moroccan Society of Rheumatology (Registre des Biothérapies de la Société Marocaine de Rhumatologie – RBSMR) is a registry for biological therapies in rheumatic diseases established by the Moroccan Society of Rheumatology. It is a historical, prospective, and multicenter registry that includes 10 departments of rheumatology across university medical centers. The patients recruited in the registry were over 18 years old. They had been diagnosed with rheumatoid arthritis (RA) or spondyloarthritis (SpA), and were receiving either initiation or ongoing biological drug therapy at various university medical centers in Morocco. The inclusion period lasted from May 2017 to January 2019, with a follow-up duration of 3 years. The number of included patients was 440, of whom 419 were validated (225 RA/194 SpA).
The primary objective of the RBSMR registry was to assess the tolerability of biological drug therapy in patients with RA or SpA treated in rheumatology settings. The secondary objectives included identifying the most common side effects encountered in daily practice, evaluating the effectiveness of biological agents in rheumatology, and assessing the impact of biotherapies on the patients’ quality of life. The details of the data collected have been published previously [5].
Study design
We conducted a prospective historical cohort study using the RBSMR database, which included 194 patients with ankylosing spondylitis (AS; according to Assessment in SpondyloArthritis international Society [ASAS] criteria) and 225 patients with RA (according to American College of Rheumatology/European Alliance of Associations for Rheumatology [ACR/EULAR] criteria). The study was designed to describe the frequency of paradoxical effects under biologic disease-modifying antirheumatic drugs (bDMARDs) over a 36-month follow-up period in patients with RA and AS included in the RBSMR, and to analyze associated characteristics.
Data collection
The occurrence of a paradoxical event was investigated at the 3-month, 6-month, 12-month, 18-month, and 36-month visits. Information regarding the date of the paradoxical effect, its nature, the type of biological agent involved, and the management of this reaction was collected. For each patient diagnosed with a paradoxical reaction induced by biological treatment, we analyzed the initially collected data from the RBSMR registry. This included the patient’s medical history, demographic, clinical, and biological characteristics (Disease Activity Score for 28 joints based on the C-reactive protein level [DAS28-CRP], Ankylosing Spondylitis Disease Activity Score based on the C-reactive protein level [ASDAS-CRP]) recorded before bDMARD initiation, immunological factors (antinuclear antibodies – ANA), genetic factors (HLA-B27 typing), and associated treatments. We compared the two groups with and without paradoxical effects and sought factors associated with the occurrence of this paradoxical reaction.
Statistical analysis
The statistical analysis was conducted using the RBSMR database with a 36-month follow-up, while the statistical analysis was performed using Jamovi software, version 2.3.21. The Kolmogorov-Smirnov test was used to assess the homogeneity of variables. Patient data were presented as mean and standard deviation for normally distributed variables, while non-normally distributed data were reported as medians and interquartile ranges. The prevalence of paradoxical reactions was calculated as a percentage. Differences in baseline demographic and clinical characteristics between patients with and without paradoxical effects were evaluated using Student’s t-test (for continuous variables), the Mann-Whitney test (for non-continuous variables), and the χ2 test or Fisher’s exact test (for categorical variables). We performed a univariate analysis followed by a multivariable logistic regression analysis to identify factors associated with the occurrence of paradoxical reactions; only characteristics frequently reported in the literature and those with a value of p < 0.20 in the univariate analysis were considered in the multivariable analysis. P-values less than 0.05 were considered statistically significant.
Bioethical standards
The protocol for the original RBSMR study was reviewed and approved by: Ethics Committee for Biomedical Research Mohammed V University-RABAT, Faculty of Medicine and Pharmacy of RABAT (Approval number: 958/09/19; September 11, 2019). Written informed consent for publication was obtained from the patients.
Results
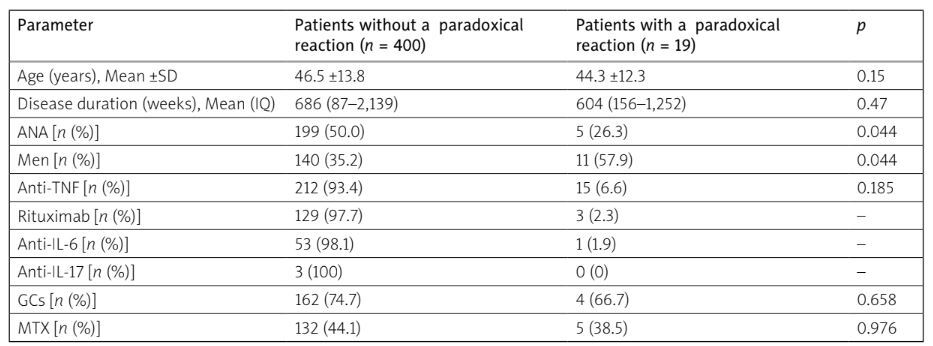
We analyzed 419 patients included in the RBSMR registry with a 36-month follow-up. Paradoxical reactions were observed in 19 patients (4.6%); 13 in patients with AS (2.9%) and 6 in patients with RA (1.7%). The average onset time of paradoxical manifestations was 30 weeks (1–144 weeks). Male gender was more common in patients with a paradoxical effect (57.9%) than in those without (35.2%), with a statistically significant difference (p = 0.04). Positive ANA results were more prevalent in the population, with a paradoxical effect (p = 0.04). Among these 19 patients with a paradoxical effect, 2 were smokers, and 1 patient had type 2 diabetes and hypertension. Two patients were on methotrexate (MTX), one on leflunomide, and one on sulfasalazine. Three patients used nonsteroidal anti-inflammatory drugs as needed. Glucocorticosteroid (GC) therapy was prescribed for three RA patients at doses ranging from 5 to 7.5 mg/day, with no statistically significant difference between the two groups with and without paradoxical effects (p = 0.65) (Table I).
Table 1
Patient characteristics*
Characteristics of paradoxical reactions
All reactions were de novo pathologies. Uveitis was the most frequent paradoxical reaction, found in 9 patients: 8 in patients with AS, and only 1 case in patients with RA. Seven cases of psoriasis were reported, including 3 in SpA patients and 4 others treated for RA. Other reactions included 1 case of pyoderma gangrenosum, 1 case of lichen, and 1 case of granulomatous dermatitis (GD). These paradoxical effects were predominantly found in men in 57.9% of cases (p = 0.044), while a high rate of positive ANA was observed in the group without paradoxical effects, reaching 50% (p = 0.044). Etanercept (ETA) was the most prescribed biological drug in patients experiencing a paradoxical reaction (52.6%), followed by adalimumab (ADA) in 21.1%, golimumab (GOL) in 15.8%, and infliximab (INF) and secukinumab (SCK) in 5.3% (Tables II and III).
Table II
Occurrence of paradoxical adverse events in SpA patients in the Moroccan biotherapy registry
[i] ADA – adalimumab, ASDAS-CRP – Ankylosing Spondylitis Disease Activity Score based on the C-reactive protein level, bDMARDs – biologic disease-modifying antirheumatic drugs, cDMARDs – conventional disease-modifying antirheumatic drugs, ETA – etanercept, F – female, GOL – golimumab, INF – infliximab, M – male, NSAIDs – nonsteroidal anti-inflammatory drugs, SCK – secukinumab, SLZ – sulphasalazine, SpA – spondyloarthritis.
Table III
Occurrence of paradoxical adverse events in RA patients in the Moroccan biotherapy registry
[i] ACPA – anti-citrullinated protein autoantibodies, ADA – adalimumab, bDMARDs – biologic disease-modifying antirheumatic drugs, cDMARDs – conventional disease-modifying antirheumatic drugs, DAS28-CRP – Disease Activity Score for 28 joints based on the C-reactive protein level, ETA – etanercept, F – female, RF – rheumatoid factor, GCs – glucocorticosteroids, LEF – leflunomide, M – male, MTX – methotrexate, RA – rheumatoid arthritis.
Evolution and treatment of reactions
Permanent discontinuation of biological treatment was recommended for all patients. Two patients were managed in a hospital setting. Additional treatments were used in the majority of patients (GCs, local treatment for uveitis).
Factors associated with the occurrence of a paradoxical reaction
In univariate analysis, the occurrence of a paradoxical effect was associated with gender (p = 0.05), positive ANA (p = 0.05), and disease activity in patients with RA (p = 0.04). In the final multivariable model, adjusted for all significant and clinically relevant variables, the paradoxical reaction was associated with disease activity (p = 0.05) with an OR = 1.03 (95% CI: 1.01–1.28; Table IV).
Table IV
Factors associated with the occurrence of paradoxical reactions under biologic therapies
Discussion
Our study revealed the occurrence of 19 paradoxical reactions under biologic therapy in the RBSMR registry, all of which were de novo pathologies. This includes 9 cases of uveitis, 7 cases of psoriasis, 1 case of pyoderma gangrenosum, 1 case of lichen, and 1 case of GD.
Uveitis
In the German pediatric registry of rheumatic diseases under biologic therapy, the occurrence of uveitis was reported in 75 out of 3,467 patients; 51 out of 2,844 patients were receiving MTX, 37 out of 1,700 patients were receiving ETA, and 13 out of 364 patients were receiving ADA [6].
They explained the high rate under ETA through selection bias. Factors associated with uveitis in this study were young age, positive ANA, and oligoarticular juvenile idiopathic arthritis (JIA). Paradoxical uveitis is not limited to anti-TNF; cases have also been reported under anti-interleukin-17 (IL-17), such as the case of uveitis under SCK noted in our results. The pathophysiological mechanism of paradoxical uveitis is based on the chronological correlation between the 2 events. Nevertheless, several hypotheses can be considered. The analysis of 794 SpA patients in the MEASURE phase 3 study found 26 cases of uveitis, with 12 considered as new cases. The majority of uveitis patterns are mediated by CD4+ Th1 (interferon γ [IFN-γ], IL-12, and TNF-α) and Th17 (IL-17, IL-21, IL-22, and IL-23) lymphocyte subtypes [7–10]. The beneficial effect of intravenous SCK has been demonstrated in cases of non-infectious uveitis [11]. Blocking the IL-17 pathway leads to an imbalance of cytokines that may explain the occurrence of paradoxical uveitis.
In the Swedish biologic registry, the incidence of anterior uveitis was 21.6 patients per year with SCK, 18 with ADA, 10 with INF, and 7.9 with ETA. Secukinumab is part of the therapeutic arsenal for spondyloarthritis. It seems to be associated with a higher risk of autoimmune uveitis compared to monoclonal anti-TNF-α and a similar risk compared to ETA. In clinical practice, SCK and ETA are associated with a higher incidence of anterior uveitis than ADA and INF [12].
Discontinuation of the medication can lead to a major flare-up of the underlying disease, which is sometimes more detrimental than the paradoxical reaction – hence the continuation of the biological agent with local treatments. However, among the 19 patients in our study, we decided to switch to another biological agent, resulting in improvement.
Psoriasis
In the German BIKER registry of JIA, psoriasis was more frequent in TNF-α inhibitor cohorts (RR 10.8, p = 0.019), particularly in the anti-TNF antibody subgroup (RR 29.8, p = 0.0009), while no significant signal was observed with ETA [13]. The occurrence of cutaneous psoriasis is most often explained by the cytokine imbalance related to the chronic inhibition of TNF-α [14, 15]. This leads to oversecretion of INF-α, which involves TH1 lymphocytes in the pathophysiology of psoriatic lesions [16]. In studies reported on psoriasis and psoriatic arthritis, the infiltration of self-reactive T cells is linked to the overexpression of the CXR3 receptor. The administration of anti-TNF-α promotes this overexpression of the receptor [17]. Interferon α activates dendritic cells and increases the expression of antigens on the skin [18]. Several studies confirm the involvement of INF-α, especially the onset of psoriasis under INF-α treatment in patients with liver disease or malignant tumors [19] and the regression of these lesions after its discontinuation [20, 21].
In the Spanish registry of inflammatory rheumatic diseases treated with biologics, 40 cases of psoriasis were reported among 5,437 patients included in the analysis, all under anti-TNF-α. Nineteen cases of psoriasis were reported with INF, 11 with ETA, and 10 with ADA, with incidence rates per 1,000 patient-years of 2.2 (95% CI: 1.4–3.4), 2 (95% CI: 1.1–3.6), and 3.2 (95% CI: 1.8–5.8), respectively; among these, 16 occurred in patients with RA (0.54%), 13 in patients with AS (1.34%), and 6 in patients with psoriatic arthritis (0.64%) [22].
In the British Society for Rheumatology Biologics Register (BSRBR), including 9,826 patients with RA under anti-TNF-α treatment, 25 patients developed psoriasis. The crude incidence rate of psoriasis was higher in patients treated with anti-TNF-α (1.04 per 1,000 person-years), and significantly higher in patients treated with ADA [23].
Pyoderma gangrenosum
Other paradoxical dermatological effects have been reported under anti-TNF-α, such as pyoderma gangrenosum, which is a neutrophilic dermatitis. Inhibition of TNF-α is thought to stimulate the synthesis of IFN-α and IL-23, leading to paradoxical reactions. A study by Guenova et al. [24] found overexpression of IL-23 in tissues of pyoderma gangrenosum.
Lichen planus
Lichenoid reactions are well described under certain biologics such as anti-TNF-α. Causality cannot be proven; however, the timing and the absence of any other identified cause strongly support a treatment-related attribution [25]. The pathophysiology of lichen planus notably involves T lymphocytes and dendritic cells implicated in the production of pro-inflammatory cytokines (including TNF-α and IFN).
Granulomatous dermatitis
Immune imbalance was implicated in the occurrence of GD under anti-TNF-α. However, cases of GD appearing under anti-TNF-α have been reported [26, 27]. Etanercept is a soluble receptor that neutralizes soluble TNF-α and binds with reduced affinity to membrane-bound TNF-α. It binds to lymphotoxin and partially respects the p75 receptor pathway; the expression of IFN-α remains free and contributes to granuloma formation, explaining the inefficacy of this agent in Crohn’s disease and refractory forms of sarcoidosis. However, some granulomatous reactions have been observed under INF or adalimumab.
The study’s limitations mainly include missing data, especially the characteristics of dermatological lesions. The strengths of the study lie in the number of patients, the duration of follow-up, and its prospective “real-world” nature. Indeed, to our knowledge, this is the first study evaluating the occurrence of paradoxical reactions in a significant number of patients with biological treatment in our country.
Conclusions
Our study suggests that there is a low prevalence of paradoxical effects in our population. These effects result from cytokine imbalance following the blockade of immune pathways by different biologics. Depending on the individual circumstances and the severity of the paradoxical event, treatment may be stopped or continued.
However, these reactions should be known and actively sought to enhance the management of patients with chronic inflammatory rheumatic diseases.