Introduction
Rheumatoid arthritis (RA) is a chronic, systemic disease of connective tissue with an autoimmune basis. The inflammatory process begins in the synovium of the joints, usually affecting them symmetrically. It leads to irreversible damage, contributing to joint pain and limited mobility, and ultimately to permanent joint damage [1]. The etiology of the disease is unknown, but it certainly depends on various factors – genetic, behavioral, and environmental. Currently, one of the most reported factors is bacterial plaque found in the periodontium [1, 2].
Periodontitis is an infectious disease. It is suggested that both diseases due to their inflammatory nature can exacerbate each other through the production of cytokines [2].
The presence of chronic inflammation in the body is crucial for the progression of both diseases. Numerous studies conducted on patients with periodontitis and RA confirm increased concentrations of pro-inflammatory cytokines and prostaglandins, reduced concentrations of tissue metalloproteinase inhibitors, and an increased number of inflammatory cells, which suggests a common pathway of both diseases [3–6]. Age above 65 years, stress, and improper diet are common risk factors for the progression of many diseases. However, there are several factors – genetic, environmental, and behavioral – common to periodontitis and RA, which are interdependent and modify their course [7]. Mutual progression of periodontitis and RA is associated with anti-cyclic citrullinated protein antibodies (ACPA).
Citrulline-rich proteins are formed in the process of post-translational deamination of arginine residues, catalyzed by the enzyme peptidyl arginine deiminase (PAD). High PAD activity, and therefore the presence of citrullinated proteins, is observed in various physiological processes, such as the inflammatory reaction or epidermal cell differentiation. Their excessive expression may also accompany many pathological processes. The presence of a citrulline residue in proteins such as type II collagen, fibrin, fibrinogen, vimentin, and a-enolase may cause an immune response directed against these proteins [8, 9].
Of the periodontal pathogens identified so far, only two – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – are associated with ACPA, due to the increased expression of the PAD enzyme. The high specificity of ACPA antibodies for the seropositive form of RA, in the case of periodontitis, leads to clear progression of both diseases [2, 9].
Virulence factors of bacteria associated with periodontitis contribute directly to chronic inflammation, independently of citrullination, by inducing the production of multifunctional pro-inflammatory cytokines: interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF), which play a key role in the pathogenesis of RA and periodontal diseases [2, 10, 11]. Their increased concentrations have been demonstrated in the synovial fluid of joints affected by the disease in patients with RA and in the fluid of periodontal pockets of patients with periodontitis [12, 13].
Interleukin-1 participates in many processes necessary to initiate and maintain the inflammatory response. It increases the production of adhesion molecules, facilitating the migration of leukocytes, stimulates the production of other inflammatory mediators and metalloproteinases, activates T and B lymphocytes, stimulates osteoclasts leading to bone resorption and affects cell apoptosis, limiting the regenerative capacity of tissues [14].
Interleukin-6, produced mainly by monocytes and macrophages, has multidirectional effects. It is involved in the differentiation of B lymphocytes and – together with IL-1 – stimulates the proliferation of T lymphocytes. Interleukin-6 may promote synovitis and damage joints by stimulating neutrophil migration, and osteoclast maturation and may mediate the formation of pannus and erosions. Interleukin-6 is also able to activate polyclonal B cells along with the production of rheumatoid factor, acute phase protein synthesis, and thrombopoiesis [15].
Tumor necrosis factor, secreted mainly by monocytes and macrophages, regulates the increase in the production of collagenases, prostaglandin E2, chemokines and cytokines, cell adhesion molecules, and factors related to bone resorption. Together with IL-1, they can also influence bone resorption by jointly activating osteoclasts [15].
The impact of periodontitis on RA is clinically evaluated in studies in terms of changing values of serum erythrocyte sedimentation rate (ESR) and Disease Activity Score with 28-joint count (DAS28) and levels of C-reactive protein (CRP) or TNF and IL-6 after clinical treatment of periodontal inflammation [2, 16]. Inflammatory mediators such as IL-6 and TNF from local periodontal damaged tissues penetrating to the systemic circulation trigger a systemic inflammatory response and promote local secretion of CRP. The prolonged inflammation ultimately results in alterations in the bone marrow with distinctive blood patterns, characterized by hyperreactive white blood cell (WBC; neutrophils and monocytes/macrophages) production. Then WBC migrate through the bloodstream, exacerbate inflammatory reactions in multiple locations, and worsen on-going infectious and inflammatory diseases.
Biologic drug therapy containing IL-6 and TNF inhibitors (TNFi) is one of the modern methods of RA treatment [16].
The aim of the study was to observe and measure periodontal tissue condition in patients with RA treated with biologic drug therapy.
Material and methods
The cross-sectional study included 50 patients with RA treated at the Department of Rheumatology, Rehabilitation, and Internal Diseases of the Medical University of Poznan. The criteria for including patients in the study were: age over 18, written consent to participate in the study, diagnosis of RA according to the current classification criteria of the American-European Consensus Group from 2010 [1], and biologic drug therapy.
Characteristics of the study group
The study group consisted of 42 women and 8 men. The ages of the patients ranged between 28 and 76 years. The mean age of the patients was 53 ±12 years. Eight patients stated that they were active smokers, which constitutes 16% of all respondents. All patients were treated with biological drugs during the study: 26 with TNFi, and 24 with IL-6 inhibitors. The duration of the disease ranged from 1 to 20 years, with an average of 8 ±5 years. Rheumatoid arthritis activity was assessed using the 28-item Disease Activity Scale (DAS28). Most patients were in remission (22) or a state of low disease activity (14). Disease activity was described as medium in 7 patients and high in 7 patients (Table I).
Table I
Characteristics of the study group (n = 50). Data are presented as mean (SD) or n (%)
The control group was an anonymous, completely healthy population of 30 blood donors (26 women, 4 men) from the Regional Center for Blood Donation and Treatment in Poznan. The mean age of patients was 41 ±12 years.
Detailed clinical and demographic data were collected from all patients. Each patient underwent a routine physical examination including a joint examination. A rheumatologist assessed disease activity during a routine monitoring visit. Then, after performing an essential examination of the oral cavity, the dentist assessed the periodontal tissues. The assessment was conducted by one doctor.
Assessment of rheumatoid arthritis activity
Rheumatoid arthritis activity was assessed using the DAS28. The index value is calculated based on the number of tender (TEN28) and swollen (SW28) joints, the erythrocyte sedimentation rate (ESR) and the patient’s assessment of the disease (Visual Analogue Scale – VAS), according to the formula: DAS28 = 0.56 √ (TEN28) + 0, 28 √ (SW28) + 0.70 Ln (ESR) + 0.014 (VAS) [68]. Disease activity was defined according to The European Alliance of Associations for Rheumatology (EULAR, formerly The European Ligue Against Rheumatism) response criteria [17].
Assessment of periodontal condition
Because the examination of patients was only possible in hospital or at the clinic, without possibility of X-ray examination, the simplest parameters were chosen to assess the periodontium status. Examination was done by one person equipped with a WHO periodontal probe. The periodontal condition was assessed by determining the values of three periodontal indexes: Approximal Periodontal Index (API), Papillary Bleeding Index (PBI) and Periodontal Screening Index or Periodontal Screening and Recording Index (PSI/PSR) [18, 19].
1. Modified Approximal Periodontal Index (mAPI, Lange 1986).
Depending on the result, the patient’s hygiene level can be determined using the indicator:
API 100–70% – improper oral hygiene,
API 69–40% – average hygiene,
API 39–25% – hygiene is quite good,
API < 25% – optimal oral hygiene.
2. Papillary Bleeding Index (PBI, Saxer & Mulehmann 1975).
Depending on the index value, the stage of gingivitis can be determined:
PBI 100–50% – severe, generalized gingivitis,
PBI 50–20% – moderate gingivitis,
PBI 20–10% – mild gingivitis,
PBI < 10% – clinically healthy gum.
3. Periodontal Screening Index PSR (Table II).
Table II
Clinical equivalents of individual PSI codes. Codes 1 and 2 indicate the presence of gingivitis, and codes 3 and 4 indicate the presence of periodontitis
Laboratory tests
To generally assess the clinical condition of patients, the following laboratory parameters were monitored: inflammatory markers: ESR and CRP, WBC leukocyte count, neutrophil count, lymphocyte count, neutrophil to leukocyte ratio (NLR), red blood cell count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, hemoglobin concentration, red cell distribution width (RDW) index, platelet count.
Statistical analysis
All statistical calculations were performed using the Statistica 10.0 computer program from Stat-Soft Polska, licensed to the Medical University of Karol Marcinkowski in Poznan. The level of statistical significance adopted in this study was p < 0.05. Numerical variables were assessed regarding compliance of their distribution with the normal distribution (Kolmogorov-Smirnov test). Because the distribution of variables deviated from the normal distribution, they were analyzed using non-parametric methods. The Mann-Whitney U test was used to compare two independent, continuous samples, and the Spearman R test was used to calculate the relationship between these variables. The relationships between categorized variables were assessed using the χ2 test.
Results
When measuring the API, which determines the level of oral hygiene based on the presence of dental plaque, in 28 participants from the study group and 14 from the control group (constituting 56% and 47% of respondents, respectively), the index value was < 25%, which means the optimal level of oral hygiene was maintained by these patients. Reasonably good and average hygiene was observed in 4 (8%) and 3 (10%) respondents, respectively. However, 15 (30%) RA patients and 13 (43%) from the control group had a result of ≥ 70%, indicating poor oral hygiene, which proves the need to further educate patients about the increased risk of periodontitis in their case. However, there was no statistically significant relationship between the API and RA activity (p = 0.768930).
The results of measuring the PBI and determining the number of bleeding spots after pressing the gingival papilla with a periodontal probe were even greater among the study group. In 19 participants, constituting 38% of all subjects from study group and 6 (20%) from the control group, the number of bleeding spots was < 10%, which characterizes healthy gums. Twelve (24%) and 15 (50%) patients respectively were diagnosed with a mild form of gingivitis, 9 (18%) and 9 (30%) from the control group with a moderate form, and severe gingivitis was diagnosed only in 10 (20%) RA patients. Since gingival bleeding can be caused by numerous factors unrelated to periodontal diseases, such as smoking, anemia or other chronic diseases, this indicator was only one of the diagnostic measurements, and the finding of no statistically significant correlation with RA activity (p = 0.895185) was confirmation of previous assumptions.
The measurement of the PSI, depending on its final value, determines the presence of gingivitis or periodontitis. In the study group, only 14% (n = 7) of participants had no symptoms qualifying for the diagnosis of gingivitis or periodontitis. In the control group it was 20% (n = 6). Considering the possible consequences of an additional source of inflammation in RA patients, even if limited only to the gum, this is a disturbingly small percentage. Index values 1 and 2 were recorded in 46% (n = 23) and 22% (n = 11) of patients, respectively, which means that a total of as many as 68% (n = 34) of the subjects had symptoms of gingivitis. In the control group it was 70% (n = 21). Values 3 and 4, assigned based on the presence of deep periodontal pockets above 3.5 mm, indicating tissue destruction characteristic of periodontitis, occurred in a total of 18% (n = 9) of patients, including 3 patients (6% of the study group) in whom the pocket depth was over 6 mm. In the control group 3 patients had periodontal pockets above 3.5 mm. Contrary to expectations, no statistically significant relationship existed between RA activity and the PSI value (p = 0.290894).
Assessment of rheumatoid arthritis activity
In the study group, most patients achieved satisfactory treatment results, showing a state of remission or low disease activity. The mean disease activity, assessed by DAS28, was 3.13 ±1.57. High disease activity (DAS28 > 5.1) was observed in only 7 patients.
Assessment of the dependence of periodontal indexes on rheumatoid arthritis activity
There were no statistically significant correlations between periodontal indexes and RA activity (Table III).
Assessment of the dependence of periodontal indexes on rheumatoid arthritis activity
There were no statistically significant correlations between periodontal indices and biological treatment (Table IV). Moreover, there were no statistically significant differences in CRP levels between the group of patients treated with TNFi and IL-6 inhibitors (p = 0.179).
Table IV
Dependence of periodontal indexes and biological treatment
Assessment of the correlation between erythrocyte sedimentation rate and C-reactive protein inflammatory markers and periodontal index values
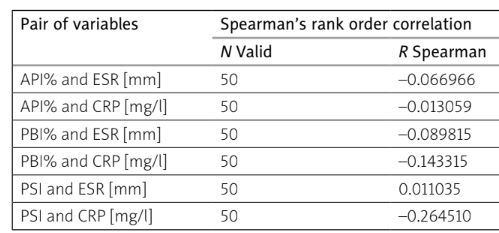
There was no statistically significant relationship between ESR and CRP values and API and PBI indicators. Since these indicators only determine the level of oral hygiene and the possible presence of gingivitis, their lack of correlation with inflammatory markers was predicted.
Also, no statistically significant correlation was found between PSI and ESR. However, the ESR index is a non-specific inflammatory marker routinely used in general examinations, and the CRP result is a more sensitive and reliable parameter.
In the case of the relationship between the PSI and the CRP parameter, a statistically significant correlation was obtained (p = 0.048837), confirming the relationship between detectable inflammation in the body and periodontitis (Table V). Moreover, in the study group the mean CRP was above the reference values, at 7.9 ±7.3 mg/l.
Table V
Assessment of the correlation between ESR and CRP inflammatory markers and periodontal index values
Assessment of the correlation between periodontal index values and erythrocyte volume distribution values and other peripheral blood count parameters
There was no statistically significant relationship between RDW values and mAPI, mSBI and PSI indicators. A correlation between PSI and lymphocyte levels was found, which was statistically significant. In the case of other parameters of peripheral blood count, no statistically significant relationships were found. The results are presented in Table VI.
Table VI
Statistical results of the correlation between the values of periodontal indices mAPI, mSBI and PSI and the values of RDW and other peripheral blood parameters
[i] API – Approximal Periodontal Index, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, HGB – hemoglobin concentration, LY – lymphocyte count, NEUT – neutrophil count, PBI – Papillary Bleeding Index, PLT – platelet count, PSI – Periodontal Screening Index, RBC – red blood cell count, RDW – red cell distribution width, WBC – white blood cells.
Assessment of correlation between periodontal index values and red cell parameters, including erythrocyte volume distribution concentration
There was no statistically significant relationship between the RDW and the values of API (p = 0.223988), PBI (p = 0.187870) and PSI (p = 0.881592), which may suggest that periodontal condition has no direct effect on red blood cell parameters.
Discussion
Biologic disease-modifying anti-rheumatic drugs (DMARDs) have revolutionized the management of RA. Biologic agents directly target molecules and cells involved in the pathogenesis of RA. Currently, five TNFi (infliximab, etanercept, adalimumab, golimumab and certolizumab pegol), an IL-6 receptor antagonist (tocilizumab), an IL-1 receptor antagonist (anakinra), a B cell depleting agent (anti-CD20, rituximab) and a T cell co-stimulation inhibitor (abatacept) have been approved for the treatment of RA. Treatment of RA with biologic DMARDs indeed leads to a better clinical remission and prognosis in patients with RA, especially in patients who are not well controlled with traditional DMARDs [20, 21]. In the case of an insufficient response to treatment, the presence of comorbidities and/or extra-articular manifestations should always be considered. As mentioned before, periodontal status may influence susceptibility to autoimmune diseases and interfere with inflammatory pathways.
There are a few hypotheses trying to explain the association between the two diseases periodontitis and RA [2]. It has been reported that individuals with periodontitis and RA suffer from systemic dysregulation of the immunological response, manifested as increased levels of IL-6 and TNF in the serum. These inflammatory mediators influence the low-grade systemic inflammation and promote local secretion of CRP (by adipocytes, vascular smooth muscle cells or gingival cells) but also can generate a distant response with distinctive blood patterns [2, 16]. However, the exact mechanism of how periodontitis triggers CRP release is still under investigation. Although CRP is recognized as an unspecific marker, the reduction of CRP level observed in clinical research stimulated further discussion on the anti-inflammatory therapy as a valuable strategy in the prevention of cardiovascular disease events, because elevated CRP values are a widely recognized cardiovascular risk factor [16, 22].
There is no doubt that in both diseases with a chronic systemic inflammatory state, risk prediction has been demonstrated when assessing basal levels of inflammation as assessed by CRP. In many studies it has been proved [1, 2, 16].
However, in our study performed on biologically treated RA patients the comparison of periodontal indexes with RA activity did not show statistically significant correlations. This result can be explained by many factors.
Firstly, the study group consisted of patients treated with biologic drugs. Oral cavity sanitation is required before starting treatment to ensure patient eligibility for such treatment [23]. A biologically treated patient, is, therefore more aware of the risk of periodontal disease, the need to maintain a high level of oral hygiene, and the need for regular check-ups with the dentist. It can also explain why the RA patients had even better clinical periodontal outcomes than healthy patients.
Studies on one biological drug have proven its negative effect on periodontium [24]. Therefore, the lack of dependence on disease activity in our studies could also be because most patients in the study group showed low activity or remission of the disease (72% in total), i.e. took lower doses of the drug. The main mechanism of biological medicines in RA is also favorable for periodontal tissue status.
Regarding the assessment of the API index, the finding of proper oral hygiene in more than half of the patients (n = 28.56%) indicates the knowledge of these patients about taking care of the appropriate condition of the oral cavity. At the same time, however, in 30% (n = 15) of patients, an API of > 70% was determined, indicating an incorrect level of hygiene requiring improvement, which may contribute to the development of periodontal disease in this group of patients.
High values of the PSI (3 or 4), synonymous with the diagnosis of periodontitis, were found in only 9 patients, and in only 3 patients, it was so advanced that the periodontal pockets reached values of > 6 mm. Worryingly, a high percentage of the examined patients were diagnosed with gingivitis (PSI level 1 or 2 in a total of 34 [68%] patients). Chapple’s research [19] has shown that the presence of untreated gingivitis is a risk factor for the development of periodontitis. Taking into account the possible coexistence of other diseases as well as genetic and environmental factors, which further modify the body’s defensive capabilities, the presence of even slight inflammation limited to the gum at the time of the examination poses a risk of developing periodontitis in the future [7, 25].
There are many reports in the widely available studies on the relationship between periodontal condition and RA activity [23–26]. However, in our study, no statistically significant correlations were found in comparing the values of periodontal indexes mAPI, mSBI and PSI with RA activity. One of the reasons for this result may be the specific characteristics of the study group. Before considering a patient eligible for biological treatment, oral cavity sanitation is required before its commencement [25]. Therefore, a biologically treated patient is more aware of the risk of periodontal disease, the need to maintain a high level of oral hygiene, and regular check-ups with the dentist. Not without significance is also the therapy which the patient receives. Beyer et al. [26] observed better periodontal condition in patients with active RA during treatment compared to patients in remission, who constituted most of the study group in their study.
In our study of the relationship between ESR and CRP inflammatory markers and periodontal indexes, no statistically significant correlation was found with mAPI and mSBI indexes determining the level of oral hygiene and the possible presence of gingivitis. Such a result confirms previous reports of elevated inflammatory markers, which are more characteristic of periodontitis than inflammation confined to the gingiva [27].
The correlation between CRP and PSI confirmed in our study indicates the impact of periodontitis on the development of systemic inflammation. The results of the study are consistent with the data from the literature [27–30]. Bolla et al. [29] found elevated serum CRP concentrations both in patients with mild and those with severe periodontitis compared to healthy controls. Also, in the study of Gupta et al. [30], higher CRP concentrations were confirmed in patients with periodontitis, which were reduced after non-surgical hygienization procedures, which indicates the importance of proper oral hygiene for the patient’s general condition.
On the other hand, in the publication of Botelho et al. [31], containing an analysis of the results of 45 studies involving the correlation of periodontitis with the results of hematological tests, higher ESR values were confirmed compared to a healthy control group. In our study, no statistically significant relationships were found between periodontitis and erythrocyte sedimentation rate. This result may be due to the specific characteristics of the study group, in which probably (despite effective therapy) the activity of the underlying disease has a more significant impact on the ESR values than the periodontal condition. No correlation between periodontal status and ESR was also found in research by Choudhury and Chakraborta [32], which suggests a lack of specificity of this indicator in periodontitis.
Limitations of study
The study has some limitations. The first is the examined group, which constituted RA patients treated biologically, when the research was starting. It makes the study more an observation than a prospective study. The second is the size of the study group.
Conclusions
Most patients in the study group had a satisfactory periodontal condition. Periodontal indexes did not correlate with RA activity or with ESR and CRP inflammatory markers, which may be due to the specific characteristics of the biologically treated study group. Elevated serum concentrations of the inflammatory marker CRP may indicate the impact of periodontal status on patients’ overall clinical condition.
However, further research is needed to determine whether the administration of biologic DMARDs is useful in reducing the production of cytokines also in the periodontium.