Introduction
Osteoporosis is the most represented metabolic bone disease and is characterized by the reduction of bone mineral density (BMD), exposing patients to a high fracture risk and disability [1].
Bisphosphonates (BPs) are the corner stone of osteoporosis treatment and are successfully exploited in reducing fracture risk. Sarcopenia is the pathological reduction of muscle masses and strength according to the latest European Working Group of Sarcopenia definition [2], and many studies highlighted its strict association with impaired bone metabolism as well as osteoporosis [3].
Although there are several effective therapies for low BMD, this is not true for sarcopenia; indeed, the only real therapeutic alternatives for the pathological reduction of lean tissue are represented by physical exercise and the implementation of a correct dietary approach [4].
Moreover, sarcopenia seems to share many pathological mechanisms with osteoporosis; thus, in this context, we decided to conduct a retrospective case-control study aimed at evaluating the effects of BPs on body composition with particular attention to lean masses.
Material and methods
Study design
We enrolled postmenopausal women from our metabolic bone diseases outpatient clinic who underwent at least two consecutive dual-energy X-ray absorptiometry (DXA) examinations concomitantly to the beginning of an antiresorptive agent.
The dual-energy X-ray absorptiometry machinery used was a Lunar Prodigy® version 1.72 which underwent daily calibration as suggested by the manufacturer. We retrospectively collected the variables over a standard 18-month observation period.
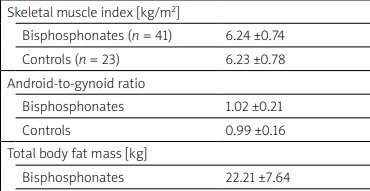
The body composition of patients and controls was compared by fat and lean masses of total body, appendicular lean mass (ALM, kg/m2) was calculated as the sum of fat-free mass minus bone mineral content of lower and upper limbs, skeletal muscle mass index (SMI, kg/m2) was calculated as ALM divided by height squared according to Baumgartner’s criteria [5].
A low muscle mass was identified for women when it was found to be 2 SD below the mean of young adults (women: < 5.5 kg/m2). The dual-energy X-ray absorptiometry scansions were performed by a trained physician with more than 10 years of experience in the field. Anthropometric variables such as weight, height, body mass index (BMI), concomitant medications, and main diseases were investigated.
Exclusion criteria
Exclusion criteria which forbid the patients from being considered in the present study were diabetes, autoimmune diseases, cancer, neurological disorders, malabsorption syndromes, inflammatory arthropathies, renal and cardiovascular impairment (NYHA III–IV).
Study populations
We divided our patients in two groups: those starting a BPs and those without impaired bone metabolism (that underwent a DXA scan for being postmenopausal) were used as control. Anthropometric variables are reported in Table I.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.4.2 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com. Age, postmenopausal age, body mass index (BMI, kg/m2), total body lean mass (TBLM, kg), total body fat mass (TBFM, kg), SMI (kg/m2) and android-to-gynoid ratio (A/G ratio) were analyzed with descriptive statistics, normality was checked with Shapiro-Wilk test and Wilcoxon test was used to compare each studied variable at baseline and after 18 months in each group. Subsequently, statistical analysis was repeated after BPs stratification.
Results
We enrolled 64 female patients: 41 starting a BP and 23 patients without treatment were used as control. Age appeared to be higher in BPs group compared to control group, BMI and postmenopausal age were similar between groups (Table I).
The skeletal muscle mass index, TBLM and TBFM appeared to be unaffected by BPs. Conversely, A/G ratio was lower in BPs group after 18 months of therapy compared to baseline observation (p < 0.05) (Table II).
Table II
Effects of antiresorptive treatments on body composition measured with dual-energy X-ray absorptiometry
The subsequent stratification for BPs failed to highlight any significant difference regarding body composition at end of study compared to baseline (Table III).
Table III
Body composition analysis according to bisphosphonates stratification
Discussion
Sarcopenia and cachexia are transversal problems in medicine and their prevalence is high among elderly patients as much as osteoporosis and fractures. The cornerstone of osteoporosis treatment passes through the fracture risk estimation, achieved with scores such as fracture risk assessment tool (FRAX) that is highly exploited in the clinical decision to identify treatment threshold. Nevertheless, most scores do not take into consideration the sarcopenia as a major risk for frailty fractures [6].
Indeed, it is well known that the reduction of muscle tissue representation is related to a higher incidence of falls and, consequently, fractures and disability. Although for osteoporosis the physician disposes of a plenty of drugs such as anti-resorptive therapies and anabolic treatments (abaloparatide, teriparatide and romosozumab) there is a lack of real therapeutic options for the pathological reduction of muscle masses [7].
Indeed, although some molecules are under study, the state of the art of the clinical management of sarcopenia is based on the implementation of physical activity and on the correct nutritional intake. Moreover, muscle and skeletal tissue cannot be considered as two separate entities, but as an endocrinologically, immunologically, and mechanically united syncytium [8].
Previous papers documented the positive effect of BPs on lean tissues after burn-injuries [9]. The BPs are molecules classified in 3 generations which differ for mechanism of action: the first generation of BPs (clodronate, etidronate, tiludronate) are incorporated as adenosine tri-phosphate analogues by osteoclasts on bone surface and induce their apoptosis, whether second and third BPs generations (nitrogen containing: risedronate, ibandronate, alendronate, pamidronate, and zoledronic acid) are capable to interfere with the mevalonate pathway by inhibiting farnesyl pyrophosphate synthase [10].
A previous paper, aimed at investigating the role of BPs alone or enriched with highly dense protein supplements in patients that underwent hip replacement after fracture, failed to highlight any significant difference in BPs treated group against control [11].
In line with these observations, the present study could not demonstrate any positive effect of antiresorptive agents on lean masses: our group of patients treated with BPs displayed any significant change in SMI compared to baseline. Furthermore, by displaying a different mechanism of action the second and third generation of BPs are held responsible for their extra-skeletal effects such as anti-tumoral activity and cardiovascular risk reduction [12].
Indeed, A/G ratio has been widely linked to an increased cardiovascular risk in literature [13] and, in accordance with this evidence [13], we highlighted the reduction of A/G ratio at end of study when compared to baseline in patients undergoing treatment with BPs.
This observation could be possibly linked to the extra-skeletal and cardioprotective effects of BPs. Nevertheless, after BPs stratification, we failed to highlight any significant difference between baseline and end of study regarding body composition variables.
Study limitations
This study has several limitations mainly bound to its retrospective nature: the age of patients appeared to be higher in BPs group compared to controls, therefore this difference may have influenced the results section; the small sample size of BPs group did not allow to highlight any significant difference among BPs group after stratification, thus not allowing to ascertain the hypothesis that second and third generation BPs may positively influence patients body composition.
Moreover, even if most of our patients were undertaking vitamin D orally, any information was available regarding its punctual dose and 25(OH)D sera levels variations during the observation period.
Indeed, adding other information such as waist-to-hip and albumin/globulin ratio may have helped strengthen the evidence that BPs may act by reducing A/G ratio and define patients nutritional status at baseline.
Conclusions
The observations made in this study did not evidence any muscle-sparing effect of BPs on lean tissues, however the improvement of A/G ratio testify that BPs may act on patients’ body composition.
Further larger studies are needed to ascertain the real effect of BPs on body composition and whether A/G ratio improvement in these subjects may reduce cardiovascular events.