Introduction
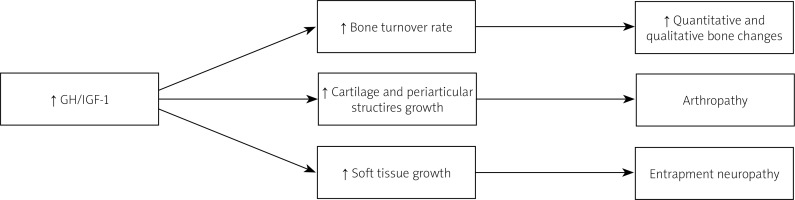
Acromegaly is a rare, chronic and disabling disease characterized by excessive growth hormone (GH) production and secretion usually caused by a pituitary adenoma (somatotropinoma), which results in elevated circulating levels of GH and insulin-like growth factor-1 (IGF-1) [1, 2]. The prevalence of acromegaly is estimated at 70 cases per million and yearly 3 to 4 new cases per million are reported [1, 3] with similar frequencies in males and females.
The diagnosis is usually made in patients between 30 and 50 years of age, but it is possible at any age. When GH hypersecretion occurs before puberty, i.e. before the closure of epiphyses by sex hormones action, it leads to excessive linear bone growth and manifests as gigantism (acrogigantism). The diagnosis is often delayed and established 5–10 years after the onset of symptoms. Such diagnostic delay leads to various complications and increased morbidity and mortality.
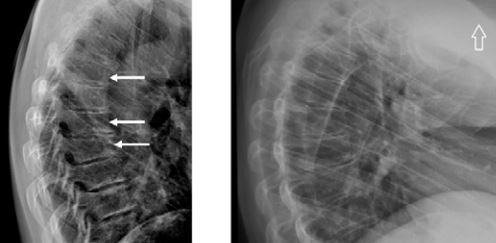
Active acromegaly results in many metabolic, cardiovascular and respiratory complications and increased risk of neoplasia, which are responsible for the higher mortality rate in this group of patients compared to the general population. Skeletal complications are frequent and they significantly reduce the quality of life of acromegaly patients causing high morbidity. The most typical musculosceletal involvement are: osteoarthritis (OA), vertebral fractures (VFs) and carpal tunnel syndrome (CTS) (Fig. 1) [1].
Growth hormone and IGF-1 are important regulators in human skeletal development throughout life, and by stimulating longitudinal bone growth in children, they determine both the accrual and the preservation of peak bone mass [4]. It was believed that due to the anabolic effects of GH on most tissues, including bones, the fracture risk in acromegaly is not increased [5]. However, the excess of GH leads to abnormalities in both cortical and trabecular bone architecture and in consequence decreased bone strength, irrespective of normal or high bone mineral density (BMD). Therefore, the risk of VFs was found to be increased [2, 6]. That is mostly due to accelerated bone turnover as reflected by elevated bone turnover markers and histomorphometric analyses of bone tissues.
Diagnosis of acromegaly
Excessive action of growth hormone and IGF-1 on various organs and tissues leads to numerous characteristic signs and symptoms in patients with acromegaly:
coarsening of facial features with an enlarged nose, auricles, mandible, frontal bossing,
tongue enlargement and separation of teeth,
deepening of the voice,
headaches,
visual field impairment,
excessive sweating,
soft tissue swelling,
skin thickening, skin tags,
prominent enlargement of hands and feet,
carpal tunnel syndrome,
bone and joint pain and bone deformations.
An examination of patients’ old photographs might be useful to identify typical changes at the early stages of the disease, when physical changes are still subtle or they are inconclusive.
In most cases acromegaly is caused by large pituitary tumors (macroadenomas) and their compression on remaining pituitary tissue may result in hypopituitarism, manifested by hypogonadism, secondary thyroid and/or adrenal insufficiency. Hypogonadism is detected in approximately 50% of patients and could also be produced by pituitary stalk compression or co-secretion of prolactin by the tumour (hyperprolactinemia). Hypopituitarism requires proper hormonal replacement. Complications of acromegaly (increased risk in patients with active disease) include:
cardiovascular:
metabolic:
respiratory:
neoplastic:
skeletal (see Table I).
All these comorbidities not only significantly reduce the quality of life, but also contribute to a 1.2–3.3-fold increase in mortality rate in these patients compared to the general population [7]. These deleterious effects can be partially reversed by achieving biochemical control and treating disease complications as soon as possible [8].
Table I
Skeletal complications in acromegaly
If acromegaly is suspected, a biochemical workup is indicated and serum IGF-1 is the most useful parameter as a screening test. Neurosurgical intervention i.e., endoscopic or microscopic transsphenoidal surgery is the treatment of choice in most cases. The normalization of GH and IGF-1 levels may lead to the subsidence of certain symptoms such as headaches, increased sweating, hypertension and impaired glucose tolerance.
Unfortunately, many complications seem to be permanent and irreversible and might persist despite adequate biochemical control. These include bone and joint deformities and increased risk of fractures. If residual disease is observed, further treatment with repeated surgical intervention, medical management, or radiotherapy should be considered.
Bone structure in acromegaly
Histomorphometric bone parameters analyzed in acromegalic patients with VFs and normal BMD showed increased cortical thickness and porosity and reduced trabecular thickness with increased trabecular separation compared to healthy control subjects [9, 10]. These abnormalities still persisted after achieving control of acromegaly with normalization of bone turnover. Severe osteoblastic dysfunction seems to be the main factor of bone disease in patients, even those with controlled acromegaly [9, 10].
Patients with acromegaly have increased trabecular bone fragility with a high prevalence of VFs despite normal BMD [11–14]. In most patients, BMD is normal or increased because the GH excess increases bone mineral content more than the bone area. The GH and IGF-I excess disrupts the trabecular microarchitecture whereas cortical bone density increases as periosteal ossification is stimulated by GH [4].
Dual-energy X-ray-absorptiometry (DXA) scanning does not differentiate between cortical and trabecular bone and the results vary depending on the ratio of these bone compartments within each site. Consistently, higher BMD was reported at the femoral neck, which is rich in cortical bone, in patients with acromegaly as compared to healthy controls [15].
On the other hand, BMD in the lumbar region may be overestimated in the presence of degenerative joint disease with osteophyte formation and facet-joint hypertrophy. Furthermore, the fracture risk assessment (FRAX) tool does not help to estimate the VF risk, since it does not include acromegaly as a secondary cause of osteoporosis [16].
The trabecular bone score (TBS) is a relatively novel tool that evaluates pixel grey-level variations in the lumbar spine DXA image. It does not increase radiation exposure since the values are obtained by analyzing the classical DXA scan [17, 18]. Higher TBS values indicate a correct trabecular bone architecture, while lower values suggest abnormal and fragile microarchitecture. Patients with acromegaly were found to have lower TBS values than healthy controls, which might reflect architectural bone changes in acromegaly [17, 18]. These abnormalities persisted even in well-controlled disease [18].
As expected, hypogonadal acromegalic patients had lower TBS values compared to eugonadal acromegaly subjects but their BMD is usually lower as well [18, 19]. Thus, in clinical practice, classical DXA measuring BMD only is of limited value in acromegaly and adding TBS calculation might help to better predict VF risk in those patients [19].
Notably, the impact of GH excess on the trabecular microstructure is partially independent of gonadal status, which has been demonstrated in multiple studies [17, 18, 20]. Thus, even eugonadal acromegaly patients have been shown to have disrupted bone microarchitecture, lower bone strength and trabecular abnormalities. Both cancellous and cortical bone compartments are affected by GH excess, and increased cortical porosity, pore volume and decreased cortical density were noted [2, 6, 9, 21, 22].
Because GH excess increases bone turnover, it contributes to increased fracture risk of vertebras, where trabecular, more metabolically active bone dominates [9]. Long bone fragility is mainly determined by the properties of the cortical bone, which is impacted differently by GH and impaired to a lesser extent than the trabecular bone, plausibly due to less active bone turnover. Furthermore, GH excess results in periosteal bone formation and in turn increase in cortical bone mass. This may partially counteract the deleterious increase in cortical porosity [23].
Given the increased risk of VFs in acromegaly and limitations of DXA in predicting them, it is suggested to implement screening by lateral conventional radiographs for VFs in newly diagnosed acromegaly [24]. It is also recommended to repeat radiological examination in follow-up, e.g., at 18–24-month intervals [25]. Calculating the TBS should also be included in the assessment when available.
Effects of growth hormone hypersecretion on bone and calcium metabolism
Active acromegaly is often accompanied by mild hypercalcemia, hyperphosphatemia and hypercalciuria [26, 27]. Despite the increased activity of α1-hydroxylase in the kidneys due to the GH excess, acromegaly patients often suffer from vitamin D deficiency [2, 26, 28]. Insufficient supply of vitamin D and its lower bioavailability at the a tissue level, plausibly caused by an increase in vitamin D binding protein concentration, may also play a role in bone abnormalities in acromegaly [29, 30]. Interestingly, it does not seem to be affected during long-term treatment with somatostatin receptor ligands (SRLs) [31].
Growth hormone excess also influences the secretion of PTH by prolongations of pulse duration and increase in pulse mass, without significant differences in baseline PTH compared to healthy controls [32]. The exact physiological consequences of these effects for bone metabolism remain unclear [33].
Achieving biochemical control of acromegaly is followed by a significant and sudden decrease in serum calcium and phosphorus levels and the normalization of hypercalciuria, however, the impact of these changes on skeletal function is still being researched [27, 34].
Fracture risk
The abnormalities in bone structure caused by GH excess predispose acromegaly patients to VFs [6]. The risk is 3- to 8-fold greater in patients with acromegaly than in healthy controls [15]. It is estimated that up to 33% of patients with newly diagnosed acromegaly have a pre-existing VF [35]. However, during long-term follow-up that number rises to 60% despite good biochemical control of acromegaly and 46–71% of these patients suffer from multiple VFs [13, 36]. The increased prevalence of VFs concerns pre-menopausal women and males [12–14, 16, 37].
Subjects with a history of long-lasting active acromegaly, with a long diagnostic delay, hypogonadism, and higher BMD Z-score at the lumbar region and femoral neck had a higher prevalence of fractures [14]. Moreover, during a 3-year observation, it was demonstrated that 42% of acromegaly patients suffered from a new VF compared to 3% in the control group [38]. The disruption of microarchitecture persisted despite obtaining biochemical control of acromegaly. A significant percentage of patients experienced progression of VFs, even those a good control of acromegaly (20–35%) [39, 40].
Thus, there is a need to assess VFs not only in newly diagnosed acromegaly patients but also in individuals cured of acromegaly or well controlled by pharmacological treatment (Fig. 2 A, B).
Fig. 2
Vertebral X-ray of a 48-year-old woman with acromegaly. Osteoporosis. Accentuated thoracic kyphosis with three wedge-shaped vertebrae at the top of the kyphosis with a moderate decrease of the anterior vertebral heights (white arrows) (A). Vertebral X-ray of a 28-year-old women with 10-year history of active acromegaly without adequate biochemical control despite multiple treatment modalities (neurosurgery, radiotherapy, pharmacological treatment). Only after initiating second generation SRLs was biochemical control achieved. Accentuated thoracic kyphosis and wedge-shaped thoracic vertebrae with decreased anterior height of vertebral bodies. Small osteophytes (B).
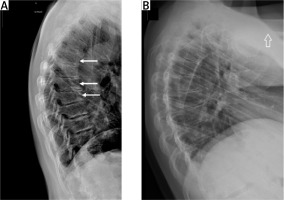
Many authors have reported that the risk of VFs was correlated with the time of active disease, male sex, hypogonadism, hypoadrenalism treated with glucocorticosteroids, elevated serum bone turnover markers, low levels of vitamin D, and a pre-existing VF [11, 13, 15, 38, 41].
Risk factors for vertebral fractures in patients with acromegaly include:
longer duration of undiagnosed active disease,
uncontrolled disease during treatment,
multihormonal pituitary deficiency as a result of the tumour itself or its treatment (neurosurgical intervention or radiotherapy),
male sex,
hypogonadism,
hyperprolactinaemia,
hypoadrenalism treated with glucocorticosteroids (excessive doses),
elevated serum bone turnover markers,
vitamin D deficiency,
pre-existing VFs.
The GH excess influences body composition and muscle mass. Subjects with active acromegaly experience a decrease in body fat and an increase in body water and lean body mass. Achieving disease control causes a reversal of these changes. Of note, muscle strength often worsens in active acromegaly despite increased muscle mass [42–44].
Some other factors might lead to limited physical activity or disability of patients with acromegaly, for example visual disturbances caused by pituitary tumor expansion, neurosurgical intervention, radiotherapy or long-standing hypertension and diabetes. Coexisting diseases such as diabetes mellitus or inadequate treatment of hypopituitarism may contribute to the disruption of skeletal structure and prevent its improvement after achieving acromegaly control [6, 41, 45].
The treatment of acromegaly with SRLs decreases the risk of VFs not only by normalizing GH and IGF-1 but also by the direct effect on bone cells via the somatostatin receptors [24]. Also, the treatment modality in acromegaly refractory to first-generation SRLs may impact the VF risk. Chiloiro et al. [46] found that active acromegaly treated with pasireotide was associated with lower VF risk compared to treatment with a GH-receptor antagonist (pegvisomant).
In patients with acromegaly, the indications for use of antiresorptive treatment remains unresolved. Antiresorptive drugs have been shown to reduce VF risk in subjects with active acromegaly but not in controlled disease [41], consistently with the thesis that in active acromegaly osteoclastogenesis prevails and in long-term controlled disease, impaired osteoblastogenesis becomes the main issue [47].
Furthermore, several authors have suggested that antiresorptive treatment should be considered in cases with multiple or moderate to severe pre-existing VFs or in refractory acromegaly that remains uncontrolled for at least one year. That was based on a finding that the risk of VFs in controlled acromegaly was correlated with pre-existing VFs [38, 39].
Acromegalic arthropathy
Patients with acromegaly are particularly prone to osteoarthritis (OA), which significantly worsens their well-being and quality of life. Up to 70% of patients with acromegaly present signs and symptoms of OA at diagnosis. Moreover, in patients cured of acromegaly or achieving long-term disease control, the prevalence of arthropathy is still increased up to 12-fold compared to healthy controls [48, 49]. This makes OA one of the main complications of acromegaly [50].
Acromegalic arthropathy differs from primary OA, mostly by causing chondrocyte and synovial cell hypertrophy and ectopic chondrogenesis [2]. Additionally, OA in acromegaly is associated with known primary OA risk factors such as female sex, older age and higher BMI [51].
Osteoarthritis begins in the active phase of acromegaly, with cartilage and soft tissue hypertrophy. It results in typical imaging findings, such as widening of joint spaces, osteophytosis and hypertrophy of the surrounding soft tissue, with cartilage hypertrophy, maintained also during long-term remission [52–54]. Transient GH/IGF-1 hypersecretion causes mainly bone and cartilage formation and protects against cartilage loss. Importantly, acromegalic patients still experience progressive OA despite adequate biochemical control [47].
Moreover, control achieved with SRLs was associated with a significantly greater progression of OA than successful surgical treatment. This observation may be related to suboptimal disease control during pharmacological treatment, even in patients meeting the current criteria of biochemical control. Another possibility includes an IGF-1-independent effect of SRLs on chondrocytes, as they have been reported to have a direct, local, mostly inhibitive influence on cartilage [49, 55].
In a study evaluating acromegalic arthropathy using MRI, a unique phenotype was found [56]. Compared to subjects with primary OA, patients with acromegaly had thickened cartilage. Additionally, in patients with active disease increased cartilage T2 relaxation times were observed compared to patients with controlled disease, reflecting their higher cartilage water content. It also showed that changes in acromegalic OA might be partially reversible due to an edema component, which decreases after achieving biochemical control of acromegaly [54].
Even though one of the most common complaints is the enlargement of hands and feet, data regarding OA of those sites are scarce. However, over 90% of patients show radiographic signs of OA at any peripheral joint site and most of them suffer from generalized OA, including multiple joints in hands in more than 80% of patients [58]. Previously, the most frequently reported OA changes concerned knees and hips. Additionally, radiographic OA of the first metatarsophalangeal joint (MTP1) might be as prevalent as radiographic knee OA (approximately 50% of patients), whereas radiographic glenohumeral OA might be as common as hip OA (40%) [56]. Pain or stiffness is most frequently reported in knees, hands, shoulders and hips. The symptoms and radiological changes often persist despite achieving acromegaly control.
There are no guidelines concerning optimal acromegalic OA management. No specific treatment that would allow for preventing or delaying OA has been developed. The most important factors remain the early diagnosis and the adequate biochemical control of acromegaly. The general symptomatic treatment used for primary OA should be implemented, including physiotherapy and analgesic agents [40, 50]. Currently, the number of papers concerning the orthopedic treatment of OA in acromegaly is scarce and those are mostly limited to a series of case reports. Regarding hip OA in acromegaly, total hip arthroplasty seems to be a promising option [57]. It should be noted, however, that none of the treatment strategies have been formally studied in acromegaly.
The bone and joint abnormalities described above are summarized in Table I.
Conclusions
The GH and IGF-1 excess in acromegaly has a deleterious impact on skeletal health and increases VFs and OA risk. The changes are partially irreversible despite achieving biochemical remission of acromegaly.
However, adequate biochemical control of acromegaly is necessary to reduce VFs risk, along with the proper substitution of coexisting hypogonadism, hypothyroidism, hypoadrenalism and vitamin D deficiency.
Antiresorptive treatment should be considered in patients with active acromegaly in whom reaching GH and IGF-1 normalization is challenging, especially in those with pre-existing VFs or untreated hypogonadism.